Today representatives of several venture and enterprise companies from US have visited the Center: Tamira Davis (EnterpriseWorks Chicago, University of Illinois), Ronnie Gist (Maryland Technology Enterprise Institute) and Jeffrey Margolis (Illinois Science and Technology Coalition).
Archive for September 30, 2014
Representatives of several venture and enterprise companies from US
International scientific conference “Science of Future”

Today participants of the International scientific conference “Science of Future” visited the Center.
Harbin Polytechnic University
School for Young Scientists

P.M. Tolstoy has participated in the School for Young Scientists “Magnetic Resonance and Magnetic Phenomena in Chemical and Biological Physics” (Novosibirsk, 7-11.09.2014) where he gave a lecture titled “Cooperativity of Functional Hydrogen Bonds in Active Sites of Enzymes: NMR Study of Model Systems”.
J. Molec. Catalysis A 2014
E.A. Valishina, M.F.C. Guedes da Silva, M.A. Kinzhalov, S.A. Timofeeva, T.M. Buslaeva, M. Haukka, A.J.L. Pombeiro, V.P. Boyarskiy, V.Yu. Kukushkin, K.V. Luzyanin
“Palladium-ADC complexes as efficient catalysts in copper-free and room temperature Sonogashira coupling”
J. Molec. Catalysis , 2014, 395, 162-171
DOI:10.1016/j.molcata.2014.08.018
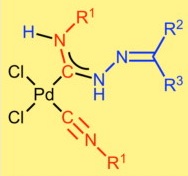
The metal-mediated coupling between cis-[PdCl2(CNR1)2] [R1 = cyclohexyl (Cy) 1, t-Bu 2, 2,6-Me2C6H3 (Xyl) 3, 2-Cl-6-MeC6H3 4] and hydrazones H2NN=CR2R3 [R2, R3 = Ph 5; R2, R3 = C6H4(OMe-4) 6; R2/R3 = 9-fluorenyl 7; R2 = H, R3 = C6H4(OH-2) 8] provided carbene complexes cis-[PdCl2{C(N(H)N=CR2R3)=N(H)R1}(CNR1)] (9–24) in good (80–85%) yields. Complexes 9–24 showed high activity [yields up to 99%, and turnover numbers (TONs) up to 3.7 × 104] in the Sonogashira coupling of various aryl iodides with a range of substituted aromatic alkynes without the need of copper co-catalyst. The catalytic procedure runs at 80 °C for 1 h in EtOH using K2CO3 as a base. No formation of homocoupling or acetylene decomposition products was observed. Designed copper-free Sonogashira system can also run at room temperature giving target products with yields up to 87% and TONs up to 87.
Eur. J. Inorg. Chem. 2014
I.I. Eliseev, P.V. Gushchin, Yi.-A. Chen, P.-T. Chou, M. Haukka, G.L. Starova, V.Yu. Kukushkin
“Phosphorescent Pt(II) Systems Featuring Both 2,2’-Dipyridylamine and 1,3,5-Triazapentadiene Ligands”
Eur. J. Inorg. Chem. 2014 , 4101-4108
DOI:10.1002/ejic.201402364
The treatment of cis-[Pt(dpa)(RCN)2][SO3CF3]2 {dpa = 2,2′-dipyridylamine, R = Me, Et, CH2Ph, Ph; [2a–d](OTf)2} (OTf = SO3CF3) with 2 equiv. of N,N′-diphenylguanidine [NH=C(NHPh)2] in CH2Cl2 solutions at room temp. for 16 h gives [Pt(dpa){NH=C(R)NC(NHPh)=NPh}][SO3CF3] {[3a,b,d](OTf)} as the addition products and [Pt(dpa){NH=C(R)NHC(R)=NH}][SO3CF3]2 {[4a,b](OTf)2} as the tailoring products. The formulation of complexes [3a,b,d](OTf) and [4a,b](OTf)2 was supported by satisfactory C, H, and N elemental analyses and agreeable high-resolution ESI-MS, IR, and 1H (including 1H–1H COSY experiments) and 13C{1H} NMR data. The structures of all of the platinum species were determined by single-crystal X-ray diffraction. The resultant complexes are nonemissive in solution, mainly because of the interaction between the empty dz2 orbital in a square-planar configuration and solvent molecules. However, in the solid state, complexes [3a,b,d](OTf) exhibit strong phosphorescence with quantum yields (peak wavelength) of 0.23 (490 nm), 0.27 (483 nm), and 0.20 (532 nm), respectively.
August 2014
Total in August 602 service applications were carried out.
All together measured:
- 567 1H spectra
- 201 13C spectra
- 107 DEPT spectra
- 2 COSY spectra
- 14 NOESY spectra
- 39 31P spectra
- 38 19F spectra
57 applications were carried out which jointly took 950 hours of measurements.