D.S. Ryabukhin, L.Yu. Gurskaya, G.K. Fukin, A.V. Vasilyev
“Superelectrophilic activation of N-aryl amides of 3-arylpropynoic acids: synthesis of quinolin-2(1H)-one derivatives”
Tetrahedron Lett., 2014, 70, 6428-6443
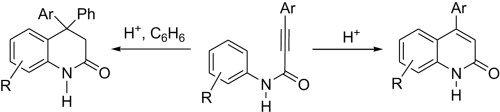
The superelectrophilic activation of N-aryl amides of 3-arylpropynoic acids by Bronsted superacids (CF3SO3H, HSO3F) or strong Lewis acids AlX3 (XјCl, Br) results in the formation of 4-aryl quinolin-2(1H)-ones in quantitative yields. The vinyl triflates or vinyl chlorides may be formed as additional reaction products. The investigated amides in reactions with benzene give 4,4-diaryl 3,4-dihydroquinolin-2-(1H)-ones under the superelectrophilic activation. 4-Aryl quinolin-2(1H)-ones in POCl3 are converted into 4-aryl 2-chloroquinolines. 4-Fluorophenyl-4-phenyl 3,4-dihydroquinolin-2-(1H)-one give N-formylation products in a yield of 79% under the VilsmeiereHaack reaction conditions.