Dz.N. Konshina, A.V. Furina, Z.A. Temerdashev, A.A. Gurinov, V.V. Konshin
“Immobilization of Guanazyl Functional Groups on Silica for Solid-Phase Extraction of Metal Ions”
Analytical Lett. 2014, accepted
DOI: 10.1080/00032719.2014.917421
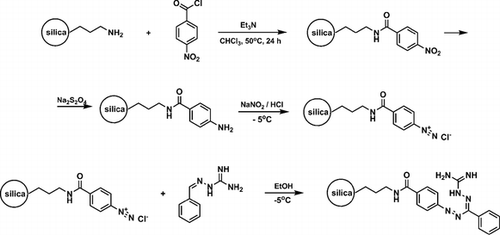
Guanazyl groups were grafted on silica gel by 4-nitrobenzoyl chloride acylation, sodium dithionite reduction, diazotation, and reaction with 2-benzylidenehydrazinecarboximidamide. The modified silica gel was used for separation and preconcentration of Cu(II), Ni(II), Cd(II), and Co(II). Quantitative extraction of the ions was achieved after 30 min and at the optimal pH of 7.5-8 at an initial concentration of 2 mg L−1. Analysis of metal sorption isotherms allowed estimation of the sorbent’s interaction efficacy under static conditions at optimal pH. Distribution coefficients were determined to be 3 ± 0.3 L g−1 for Ni(II), 3 ± 0.3 L g−1 for Co(II), 1.6 ± 0.2 L g−1 for Cd(II), and 4.6 ± 0.4 L g−1 for Cu(II) at 20-60 µg analyte. The applicability of pseudo-second order kinetic equations for metal sorption kinetics description was investigated. Chemically modified silica was used for solid-phase extraction of the metal ions to improve the detection limit using X-ray fluorescence spectrometry. The method was employed for the determination of Cu(II) in water with a low limit of detection, high accuracy, and good precision.