M.T. Dau, J.R. Shakirova, A.J. Karttunen, E.V. Grachova, S.P. Tunik, A.S. Melnikov, T.A. Pakkanen, I.O. Koshevoy
“Coinage Metal Complexes Supported by the Tri- and Tetraphosphine Ligands”
Inorg. Chem., 2014, ASAP
DOI: 10.1021/ic500402m
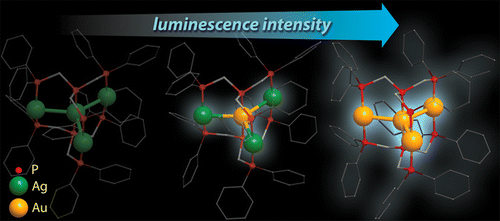
A series of tri- and tetranuclear phosphine complexes of d10 metal ions supported by the polydentate ligands, bis(diphenylphosphinomethyl)phenylphosphine (PPP) and tris(diphenylphosphinomethyl)phosphine (PPPP), were synthesized. All the compounds under study, [AuM2(PPP)2]3+ (M = Au (1), Cu (2), Ag (3)), [M4(PPPP)2]4+ (M = Ag (4), Au (5)), [AuAg3(PPPP)2]4+ (6), and [Au2Cu2(PPPP)2(NCMe)4]4+ (7), were characterized crystallographically. The trinuclear clusters 1–3 contain a linear metal core, while in the isostructural tetranuclear complexes 4–6 the metal framework has a plane star-shaped arrangement. Cluster 7 adopts a structural motif that involves a digold unit bridged by two arms of the PPPP phosphines and decorated two spatially separated CuI ions chelated by the remaining P donors. The NMR spectroscopic investigation in DMSO solution revealed the heterometallic clusters 2, 3, and 6 are stereochemically nonrigid and undergo reversible metal ions redistribution between several species, accompanied by their solvation–desolvation. The complexes 1–3 and 5–7 exhibit room temperature luminescence in the solid state (Φem = 6–64%) in the spectral region from 450 to 563 nm. The phosphorescence observed originates from the triplet excited states, determined by the metal cluster-centered dσ* → pσ transitions.
