B. Koeppe, J. Guo, P.M. Tolstoy, G. Denisov, H.-H. Limbach
“Solvent and H/D Isotope Effects on the Proton Transfer Pathways in Heteroconjugated Hydrogen-Bonded Phenol-Carboxylic Acid Anions Observed by Combined UV-Vis and NMR Spectroscopy”
J. Am. Chem. Soc. 2013, accepted.
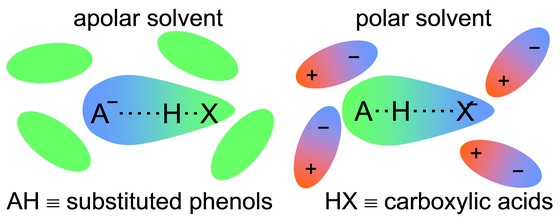
ABSTRACT: Heteroconjugated hydrogen-bonded anions A···H···X– of phenols (AH) and carboxylic/inorganic acids (HX) dissolved in CD2Cl2 and CDF3/CDF2Cl have been studied by combined low-temperature UV-Vis and 1H/13C NMR spectroscopy (UVNMR). The systems constitute small molecular models of hydrogen-bonded cofactors in proteins such as the photoactive yellow protein (PYP). Thus, the phenols studied include the PYP cofactor 4-hydroxycinnamic acid methyl thioester, and the more acidic 4-nitrophenol and 2-chloro-4-nitrophenol which mimic electronically excited cofactor states. It is shown that the 13C chemical shifts of the phenolic residues of A···H···X–, referenced to the corresponding values of A···H···A–, constitute excellent probes for the average proton positions. These shifts correlate with those of the H-bonded protons, as well as with the H/D isotope effects on the 13C chemical shifts. A combined analysis of UV-Vis and NMR data was employed to elucidate the proton transfer pathways in a qualitative way. Dual absorption bands of the phenolic moiety indicate a double-well situation for the shortest OHO hydrogen bonds studied. Surprisingly, when the solvent polarity is low the carboxylates are protonated whereas the proton shifts towards the phenolic oxygens when the polarity is increased. This finding indicates that because of stronger ion-dipole interactions small anions are stabilized at high solvent polarities and large anions exhibiting delocalized charges at low solvent polarities. It also explains the large acidity difference of phenols and carboxylic acids in water, and the observation that this differences is strongly reduced in the interior of proteins when both partners form mutual hydrogen bonds.