Julia R. Shakirova, Elena V. Grachova, Antti J. Karttunen, Vladislav V. Gurzhiy, Sergey P. Tunik and Igor O. Koshevoy
“Metallophilicity-assisted assembly of phosphine-based cage molecules”
Dalton Trans., 2014, Advance Article
DOI: 10.1039/c3dt53645a
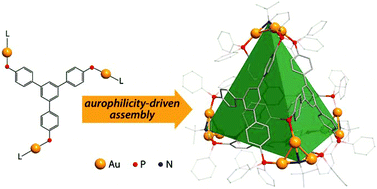
A family of supramolecular cage molecules has been obtained via self-assembly of the phosphine–gold coordination complexes following an aurophilicity-driven aggregation approach. Use of the di- (PP) or tridentate (PPP) phosphine ligands Pn (n = 2, 3) with rigid polyaromatic backbones leads to clean formation of the coordination Pn(Au(tht))nn+ species, sequential treatment of which with H2O/NEt3 and excess of H2NBut gives the finite 3D structures of two major types. The cylindrical-like hexametallic cages [(PPAu2)3(μ3-NBut)2]2+ are based on the diphosphines PP = 1,4-bis(diphenylphosphino)benzene (1), 4,4′-bis(diphenylphosphino)biphenyl (2), 4,4′′-bis(diphenylphosphino)terphenyl (3), while the triphosphine PPP (1,3,5-tris(diphenylphosphinophenyl)benzene) produces a tetrahedral dodecagold complex [(PPPAu3)4(μ3-NBut)4]4+ (4). The cages 1–4 have been studied using the ESI-MS and 1H, 31P NMR spectroscopy, and the crystal structures of 1 and 4 were determined by an X-ray diffraction study. The NMR spectroscopic investigations showed that cylindrical complexes 1–3 undergo twisting-like interconversion of the helical P↔M isomers in solution, while 4 is a stereochemically rigid compound retaining its axially chiral architecture. The difference in dynamic behaviour was rationalized using computational studies with density functional methods.