T.V. Serebryanskaya, A.S. Novikov, P.V. Gushchin, A.A. Zolotarev, V.V. Gurzhiy, V.Yu. Kukushkin
“Coupling of platinated triguanides with platinumactivated nitriles as a novel strategy for generation of dimetallic systems” Dalton Trans., 2015, 44, 6003-6011
DOI:10.1039/c4dt03870c
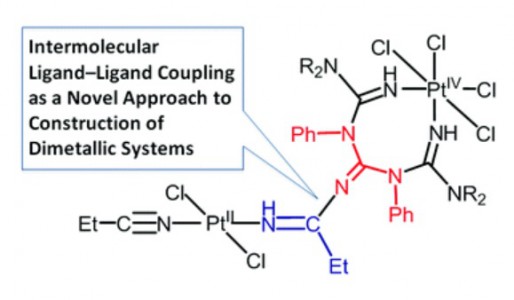
One of two PtIV-activated propanenitriles in trans-[PtCl4(EtCN)2] is involved in platinum(IV)-mediated nitrile–imine coupling with the platinum(II)-based metallacycles [PtCl2{NH=C(NR2)N(Ph)C(=NH)N(Ph)C(NR2)=NH}] [R2 = Me2 (1a), C5H10 (1b)] yielding diplatinum products, whose structures depend on molar ratios between the reactants. At a 1:1 ratio, the mixed-valence platinum(II)/platinum(IV) species [PtCl4{NH=C(NR2)N(Ph)C{=[(N(Et)C=NH)PtCl2(EtCN)]}N(Ph)C(NR2)==NH}] [R2 = Me2 (2a), (CH2)5 (2b)] were generated, whereas at a 1:2 ratio the dinuclear platinum(II)/platinum(II) complexes [PtCl2{NH=C(NR2)N(Ph)C{=[(N(Et)C=NH)PtCl2(EtCN)]}N(Ph)C(NR2)=NH}] [R2 = Me2 (3a), (CH2)5 (3b)] were obtained. In contrast to the nitrile–imine coupling observed for the platinum(IV) dinitrile complex, the reaction between the platinum(II) congener trans-[PtCl2(EtCN)2] and any one of 1a,b gives exclusively the substituted dimetallic platinum(II)/platinum(II) products [PtCl2{NH=C(NR2)N(Ph)C{=[(NH)PtCl2(EtCN)]}N(Ph)C(NR2)=NH}] [R2 = Me2 (6a), (CH2)5 (6b)] featuring platinum-containing guanidine 1 as one of the ligands. Complexes 2a,b, 3a,b, and 6a,b were characterized by elemental analyses (C, H, N), HRESI-MS, IR, 1H NMR spectroscopy, and DTA/TG. The molecular and crystal structure of 2a·2CDCl3 was additionally studied by single-crystal X-ray diffraction. Complexes 2a,b undergo further redox transformation in solutions, and single crystals of [PtCl2{NH=C(NMe2)N(Ph)C{=[(N(Et)C=NH)PtCl2(MeCN)]}N(Ph)C(NMe2)=NH}]·2CH2Cl2 (3′a·2CH2Cl2) were obtained from 2a in a CH2Cl2–MeCN–C2H4Cl2 mixture and studied by X-ray crystallography. The driving forces for the generation of diplatinum products 2 and 3 were elucidated based on a quantum-chemical study.