Сегодня ресурсный центр посетила делегация Парижского технологического института: Dr. Cecile Vigouroux, Professor Jean-Baptiste d’Espinose de Lacaillerie, Ms. Gabriela Caracaleanu
Archive for 31.10.2014
Визит делегации Парижского технологического института
Inorg. Chem. Commun 2014
M.Ya. Demakova, K.V. Luzyanin, G.L. Starova, V.Yu. Kukushkin
“Facile alternative route to cis-[PtCl2(PTA)2] and [PtCl(PTA)3]Cl (PTA = 1,3,5-triaza-7-phosphaadamantane)”
Inorg. Chem. Commun., 2014, 50, 17-18
DOI: 10.1016/j.inoche.2014.10.002
The reaction of trans-[PtCl2(Me2SO)2] with 2 equivs of 1,3,5-triaza-7-phosphaadamantane (PTA) in MeNO2 at RT furnished cis-[PtCl2(PTA)2] (1) in 87% isolated yield. Corresponding reaction of K2[PtCl4] with 1 equiv. of PTA in aqueous EtOH at RT led to [PtCl(PTA)3]Cl (2) in 84% isolated yield. Complexes 1 and 2 were characterized by elemental analyses (C, H, N), HR-ESI+/−-MS, IR, 1H and 31P{1H} NMR spectroscopic techniques, and by single-crystal X-ray diffraction for 1.
Inorg. Chem., 2014
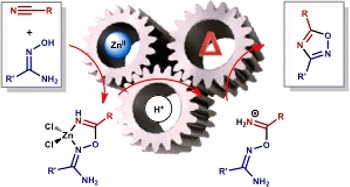
D.S. Bolotin, K.I. Kulish, N.A. Bokach, G.L. Starova, V.V. Gurzhiy, V.Yu. Kukushkin
“Zinc(II)-Mediated Nitrile−Amidoxime Coupling Gives New Insights into H+‑Assisted Generation of 1,2,4-Oxadiazoles”
Inorg. Chem., 2014, 53, 10312-10324
DOI: 10.1021/ic501333s
The cyanamides Me2NCN (1a), OC4H8NCN (1b), and PhC(═O)N(H)CN (1c) and the conventional nitriles PhCN (1d) and EtCN (1e) react with 1 equiv of each of the amidoximes R′C(═NOH)NH2 (R′ = Me (2a), Ph (2b)) in the presence of 1 equiv of ZnCl2 producing the complexes [ZnCl2{HN═C(R)ON═C(R′)NH2}] (R/R′ = NMe2/Me (3a), NMe2/Ph (3b), NC4H8O/Me (3c), NC4H8O/Ph (3d), N(H)C(═O)Ph/Me (3e), N(H)C(═O)Ph/Ph (3f), Ph/Me (3g), Ph/Ph (3h), Et/Ph (3j)) with the chelate ligands originating from the previously unreported zinc(II)-mediated nitrile–amidoxime coupling. Addition of 1 equiv of p-TolSO3H to any of one 3a–h, 3j results in the ligand liberation and formation of the iminium salts [H2N═C(R)ON═C(R′)NH2](p-TolSO3) ([4a–j](p-TolSO3)), which then at 20–65 °C spontaneously transform to 1,2,4-oxadiazoles (5a–j). As a side reaction, cyanamide derived species [4a–f](p-TolSO3) undergo Tiemann rearrangement to produce the substituted ureas R′NHC(═O)NH2 (R′ = Me (6a), Ph (6b)) and RC(═O)NH2 (R = NMe2 (6c), NC4H8O (6d), N(H)C(═O)Ph (6e)), whereas phenyl and ethyl cyanide derivatives besides their transformation to the oxadiazoles undergo hydrolysis to the parent amidoxime R′C(═NOH)NH2 (R′ = Me (2a), Ph (2b)) and the carboxamides RC(═O)NH2 (R = Ph (6f), Et (6g)). All new obtained compounds were characterized by HRESI-MS, IR, ATR-FTIR, 1H NMR, CP-MAS TOSS 13C NMR, elemental analyses (C, H, N), and single crystal X-ray diffraction for seven species (3a–e, [4b](p-TolSO3), and [4d](p-TolSO3)). Two previously unknown heterocycles 5c and 5e were isolated and characterized by elemental analyses (C, H, N), HRESI-MS, IR, 1H and 13C{1H} NMR. The observed conversion of [4a–j](p-TolSO3) to the 1,2,4-oxadiazoles uncovers the mechanism of the previously reported H+-assisted generation of these heterocycles (Augustine; et al. J. Org. Chem. 2009, 74, 5640).
Dyes and Pigments, 2014
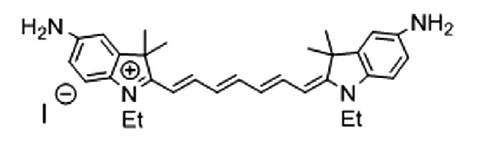
S. Miltsov, V. Karavan, M. Goikhman, I. Podeshvo, S. Gómez-de Pedro, M. Puyol, J. Alonso-Chamarro
“Synthesis of bis-aminosubstituted indocyanine dyes for their use in polymeric compositions”
Dyes and Pigments, 2014, 109, 34-41
DOI:10.1016/j.dyepig.2014.05.002
The synthesis of a set of open-chain bis-aminosubstituted cyanine dyes as well as others with cyclic fragments in the polymethine chain is presented. These dyes are suitable for the development of polymeric compositions with variable optical characteristics as they can be covalently incorporated into the polymer.
Polymer Science B, 2014
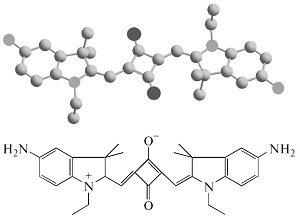
M.Ya. Goikhmana, N.P. Yevlampieva, I.V. Podeshvo, S.A. Mil’tsov, V.S. Karavan, I.V. Gofman, A.P. Khurchak, A.V. Yakimansky
“Polymers with Cyanine Chromophore Groups in the Main Chain: Synthesis and Properties”
Polymer Science B, 2014, 56, 352-359
DOI:10.1134/S1560090414030051
Polyamides containing fragments of two cyanine chromophores in the main chain are synthesized, and their viscometric and electro-optical properties in solutions, as well as their stress-strain properties in films, are investigated. It is shown that the molecular characteristics of the copolyamides are substantially affected by chromophore fragments at a content of 10 mol %, while the mechanical properties of the films are independent of the chemical structures of chromophores incorporated into polyamide chains
Экскурсия участников конференции в РЦ МРМИ
22.10 в РЦ прошла экскурсия участников 1-й междисциплинарной конференции «Современные решения для исследования природных, синтетических и биологический материалов»
Конференция 20 – 22 октября
20-22 октября сотрудники РЦ МРМИ приняли участие в работе 1-й междисциплинарной конференции «Современные решения для исследования природных, синтетических и биологический материалов». Были представлены следующие работы:
1. А.Ю. Иванов, В.В. Соколов «Использование селективных одномерных и гибридных двумерных методов спектроскопии ЯМР для определения конфигурации некоторых трициклических конденсированных производных тиазолидина с узловым атомом азота» (устный доклад).
2. М.А. Вовк, С.А. Пылаева, М. Сигалов, А. Филяровский, П.М. Толстой, «Примеры использования динамического ЯМР для определения констант заторможенного вращения вокруг связей CC и CN» (постер).
3. Е.В. Кукушкина, М.Г. Шеляпина, «Сравнение и анализ спектров ЯМР на ядрах 51V в сплавах Fe(100-x)V(x) и YFe(12-x)V(x)» (постер).
4. М.А. Вовк, А.С. Конева, Е.А. Сафонова, «Возможности по измерению диффузии в РЦ МРМИ на примере микроэмульсий» (постер).
5. С.Н. Смирнов, А.Ю. Иванов, Е.В. Грачева, И.О. Кошевой, «Изучение агрегации комплексов платины в растворе методом спектроскопии ЯМР» (постер).
6. Н.О. Саблина, В.Ю. Смутин, С.Н. Бритвин, С.Н. Смирнов, В.А. Гиндин, И.С. Игнатьев, «Изучение и молекулярный дизайн аллостерических модуляторов mGLuR5 на основе производных 1,2,4-триазин-5-онов» (постер).
Конференция 20-22 октября
Уважаемые пользователи, в связи с проведением 1-й междисциплинарной конференции «Современные решения для исследования природных, синтетических и биологических материалов», 20-22 октября 2014 года возможны задержки в выполнении заявок, т.к. сотрудники РЦ принимают активное участие в работе конференции.
Dalton Trans, 2014
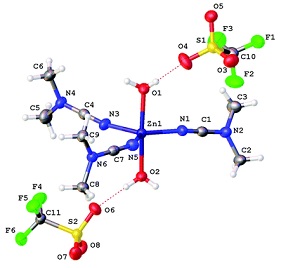
A.S. Smirnov, E.S. Butukhanova, N.A. Bokach, G.L. Starova, V.V. Gurzhiy, M.L. Kuznetsov, V.Yu. Kukushkin
“Novel (cyanamide)ZnII complexes and zinc(II)-mediated hydration of the cyanamide ligands”
Dalton Trans, 2014, 43, 15798-15811
DOI:10.1039/c4dt01812e
The cyanamides NCNR2 (R2 = Me2, Ph2, C5H10) react with ZnX2 (X = Cl, Br, I) in a 2[thin space (1/6-em)]:[thin space (1/6-em)]1 molar ratio at RT, giving a family of zinc(II) complexes [ZnX2(NCNR2)2] (R2 = Me2, X = Cl 1, X = Br 2, X = I 3; R2 = C5H10, X = Cl 4, X = Br 5; X = I 6; R2 = Ph2, X = Cl 7, X = Br 8, X = I 9; 75–92% yields). Complexes 7 and 8 undergo ligand redistribution in wet CH2Cl2 solutions giving the [Zn(NCNPh2)4(H2O)2][Zn2(μ-X)2X4] (X = Cl 10, Br 11) species that were characterized by 1H NMR, HRESI-MS, and X-ray diffraction. Halide abstraction from 1–3 by the action of AgCF3SO3 or treatment of Zn(CF3SO3)2 with NCNR2 (R2 = Me2, C5H10) leads to labile complexes [Zn(CF3SO3)2(NCNR2)3] (R2 = Me2, 12; C5H10, 13). Crystallization of 12 from wet CH2Cl2 or from the reaction mixture gave [Zn(NCNMe2)3(H2O)2](SO3CF3)2 (12a) or [Zn(CF3SO3)2(NCNMe2)2]∞ (12b), whose structures were determined by X-ray diffraction. The ZnII-mediated hydration was observed for the systems comprising ZnX2 (X = Cl, Br, I), 2 equiv. NCNR2 (R2 = Me2, C5H10, Ph2) and ca. 40-fold excess of water and conducted in acetone at 60 °C (R2 = Me2, C5H10) or 80 °C (R2 = Ph2) in closed vials, and it gives the urea complexes [ZnX2{OC(NR2)NH2}] (R2 = Me2, X = Cl 13, X = Br 14, X = I 15; R = C5H10, X = Cl 16, X = Br 17; X = I 18; R2 = Ph2, X = Cl 19, X = Br 20, X = I 21; 57–81%). In contrast to the ZnII-mediated hydration of conventional nitriles, which proceeds only in the presence of co-catalyzing oximes or carboxamides, the reaction with cyanamides does not require any co-catalyst. Complexes 1–9, 12–19 were characterized by 1H, 13C{1H} NMR, IR, HRESI-MS, and X-ray crystallography (for 1–3, 8, 9, 13–15, and 17), whereas 20 and 21 were characterized by HRESI+-MS and 1H and 13C{1H} NMR (for 20). The structural features of the cyanamide complexes 1, 2, 7, and 8 were interpreted by theoretical calculations at the DFT level.