I.A. Smetanin, M.S. Novikov, N.V. Rostovskii, A.F. Khlebnikov, G.L. Starova, D.S. Yufit
“4-Halo-2-azabuta-1,3-dienes as intermediates in the rhodium carbenoid-initiated transformation of 2-halo-2H-azirines into 2,3-dihydroazetes and 2,5-dihydrooxazoles”
Tetrahedron, 2015, 71, 4616-4628
DOI:10.1016/j.tet.2015.05.022
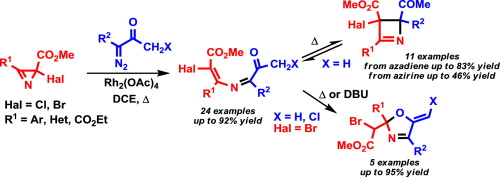
A wide range of electron-poor 4-bromo-/4-chloro-2-azabuta-1,3-dienes were synthesized by the Rh2(OAc)4-catalyzed reaction of diazo esters and diazo ketones with methyl 2-halo-2H-azirine-2-carboxylates. The E stereoselectivity with respect to the configuration of the Cdouble bond; length as m-dashC bond of the 2-azadiene moiety is in good agreement with the results of DFT (M06-2X/6-31+G(d,p)) calculations of the reaction pathway. The reaction proceeds via the formation of an azirinium ylide intermediate followed by ring opening with outward rotation of the halogen atom. Depending on the substitution pattern at C1 electron-poor 4-halo-2-azabutadienes can undergo two types of cyclization at elevated temperatures: reversible 1,4-electrocyclization to give 2,3-dihydroazetes or 1,5-exo-trig cyclization to give 5-methylene-2,5-dihydrooxazoles, both in good yields. The dihydrooxazole derivatives can be also obtained at ambient temperature under DBU catalysis.