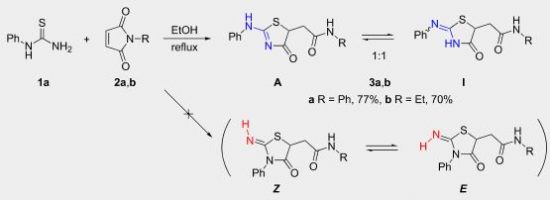
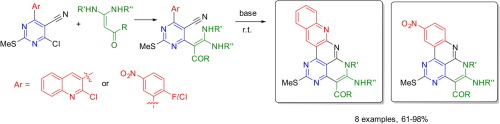
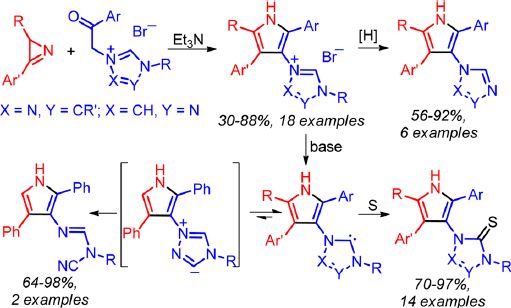
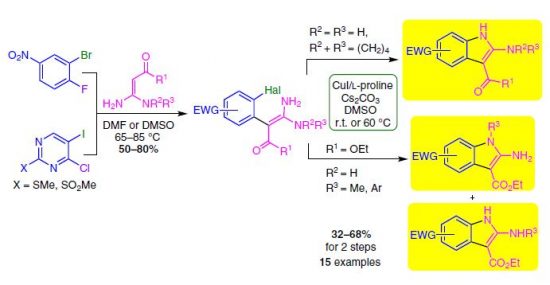
Е.А. Катленок, С.Н. Смирнов, А.Ю. Иванов, С.В. Макаренко, К.П. Балашев
“Влияние донорно-акцепторных свойств лигандов на спектроскопические и электрохимические характеристики смешанно-лигандных циклометаллированных комплексов Pt(II) и Ir(III) 2-фенилбензотиазола”
Оптика и Спектроскопия, 2017,122(3), 436-444
DOI:10.7868/S0030403417030126
Приведены результаты спектроскопических ЯМР 1Н, 13C, 195Pt, ИК, оптических и вольтамперометрических характеристик смешанно-лигандных комплексов Pt(II) и Ir(III) с металлированным 2-фенилбензотиазолом и трет-бутилизоцианидом (tBuNC), ацетонитрилом (AN), этилендиамином(En), О-этилдитиокарбонат- (Exn–) и диэтилдитиокарбонат-ионами (Dtc–). Показано, что изменение донорно-акцепторного взаимодействия с металлом лигандов tBuNC, AN, En, Exn–, Dtc– приводит к повышению энергии высшей заполненной молекулярной орбитали комплексов и сопровождается катодным смещением потенциала металл-центрированного окисления, батохромным смещением спин-разрешенного и спин-запрещенного оптических переходов переноса заряда металл-циклометаллированный лиганд и увеличением степени смешивания 1ПЗМЛ- и триплетного внутрилигандного состояния, ответственного за фосфоресценцию комплексов.