Ознакомительная экскурсия для учителей химии и биологии из школ Санкт-Петербурга и Ленинградской области.
Tag Archive for Толстой
Ознакомительная экскурсия
Dalton Trans. 2015, accepted
A. S. Antonov, A. F. Pozharskii, V. A. Ozeryanskii, A. Filarowski,
K. Yu. Suponitsky, P. M. Tolstoy, M. A. Vovk
“Ring Lithiation of 1,8-Bis(dimethylamino)naphthalene: Another Side of the ‘Proton Sponge Coin’”
Dalton Trans., 2015, accepted
DOI:10.1039/C5DT02482J
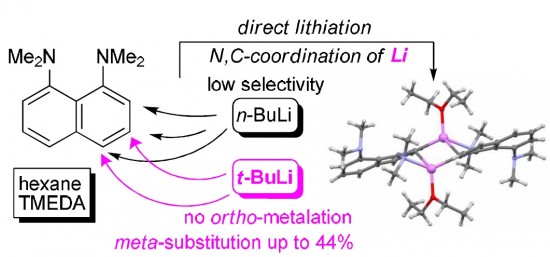
It has been found that 1,8-bis(dimethylamino)naphthalene (DMAN), unlike N,N-dimethylaniline, undergoes ring metallation in n-BuLi–TMEDA–Et2O system with low selectivity and in poor total yield. The situation is significantly improved in t-BuLi–TMEDA–n-hexane system when 3- and 4-lithium derivatives become the only reaction products in good yield. The formation of 3-Li-DMAN is especially fortunate since no method of direct meta-functionalization of DMAN has been known to date. The relative stability and structure of DMAN lithium derivatives have been examined with the help of X-ray and multinuclear NMR measurements as well as DFT calculations.
Spectrochimica Acta, Part A 2015
T. Kozlecki, P.M. Tolstoy, A. Kwocz, M.A. Vovk, A. Kochel, I. Polowczyka, P.Yu. Tretyakov, A. Filarowski
“Conformational state of β-hydroxynaphthylamides and barriers for the rotation of the amide group”
Spectrochimica Acta, Part A, 2015, accepted
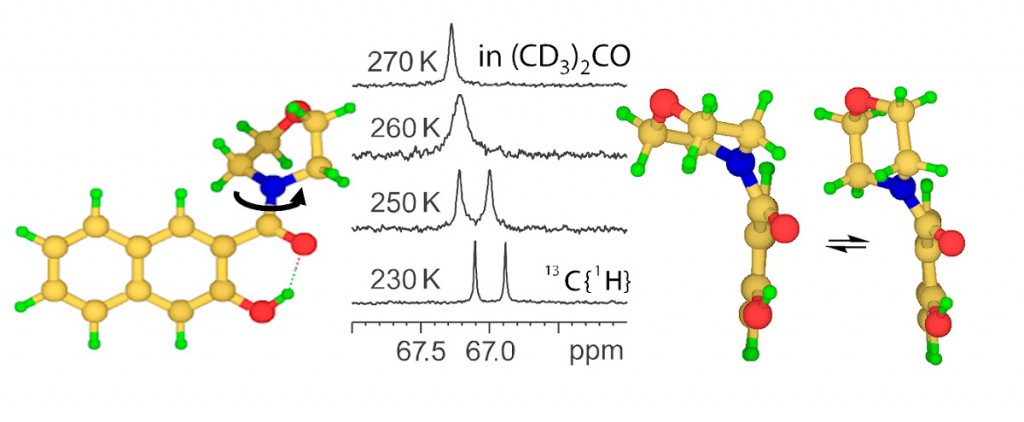
Three β-hydroxynaphthylamides (morpholine, pyrrolidine and dimethylamine derivatives) have been synthesized and their conformational state was analysed by NMR, X-ray and DFT calculations. In aprotic solution the molecules contain intramolecular OHO hydrogen bonds, which change into intermolecular ones in solid state. The energy barriers for the amide group rotation around the CN bond were estimated from the line shape analysis of 1H and 13C NMR signals. A tentative correlation between the barrier height and the strength of OHO bond was proposed. Calculations of the potential energy profiles for the rotations around CC and CN bonds were done. In case of morpholine derivative experimental indications of additional dynamics: chair-chair ‘ring flip’ in combination with the twisting around CC bond were obtained and confirmed by quantum chemistry calculations.
PCCP 2015
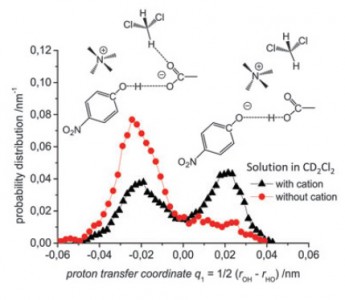
S. Pylaeva, C. Allolio, B. Koeppe, G.S. Denisov, H.-H. Limbach, D. Sebastiani, P.M. Tolstoy
“Proton transfer in hydrogen bonded complex caused by solvation shells fluctuations: ab initio MD study of anionic phenolate-carboxylic acid and neutral pyridine-carboxylic acid systems”
PCCP, 2015, accepted
DOI: 10.1039/C4CP04727C
J. Phys. Chem. A, 2014
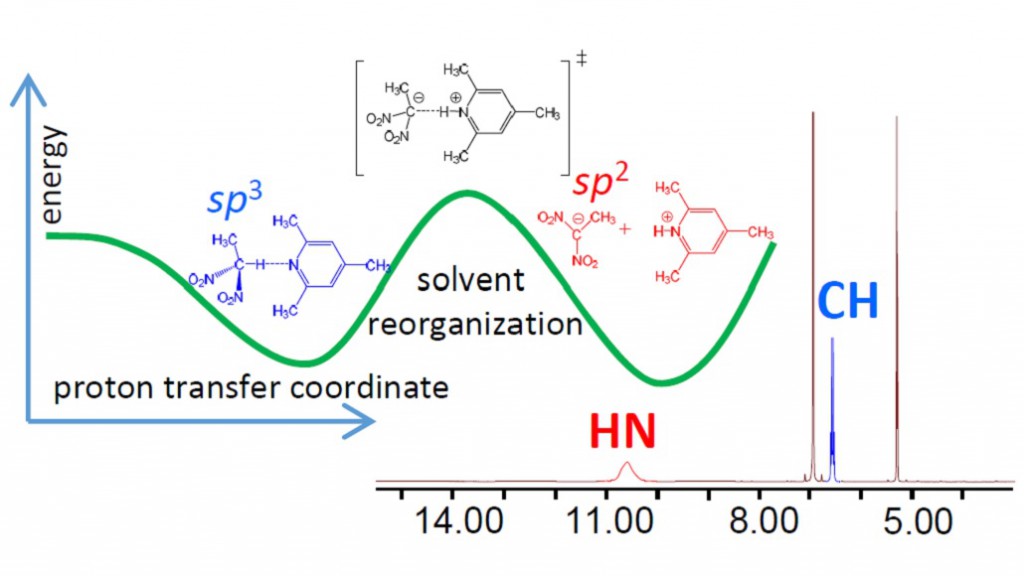
E.Yu. Tupikina, G.S. Denisov, P.M. Tolstoy
“NMR Study of CHN Hydrogen Bond and Proton Transfer in 1,1-dinitroethane Complex with 2,4,6-trimethylpyridine”
J. Phys. Chem. A., 2014, accepted
Intermolecular complex with CHN hydrogen bond formed by 1,1-dinitroethane (DNE) and 2,4,6-trimethylpyridine (collidine) dissolved in CD2Cl2 was studied experimentally by 1H NMR spectroscopy at 180-300 K. Equilibrium between molecular CH⋅⋅⋅N form and zwitterionic C−/HN+ form was detected in slow exchange regime in NMR time scale. No sign of direct C−⋅⋅⋅HN+ bond was observed; the ion pair is likely to be held by Coulomb interactions. Moreover, there are indications that the protonated base is involved in formation of homo-conjugated (NHN)+ collidine-collidinium hydrogen bonded complexes.
The reaction pathway of proton transfer in DNE-pyiridine complex in vacuum was studied computationally at B3LYP/6-31++G(d,p) level of theory. NMR chemical shifts and coupling constants were calculated for a series of snapshots along the proton transfer coordinate. While central carbon atom has pyramidal (sp3) configuration in DNE, it is flat (sp2) in DNE carbanion. As a result, the most indicative computed NMR parameter reflecting hybridization of carbon atom appeared to be 1JCC, which starts to change rapidly as soon as structure with quasi-symmetric C⋅⋅H⋅⋅N bond is reached. Couplings within the hydrogen bridge, 1JCH, 1hJHN and 2JCN, can serve as good indicators of the degree of proton transfer.
Chem. Eur. J., 2014
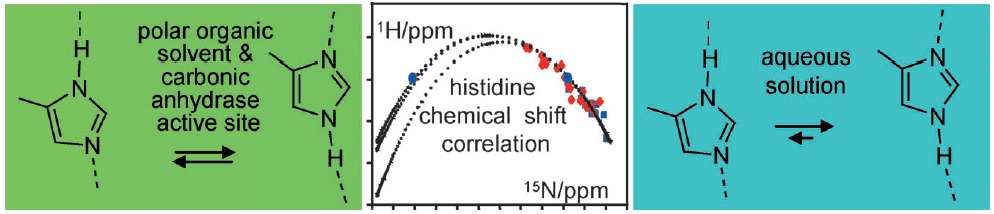
I.G. Shenderovich, S.B. Lesnichin, C. Tu, D.N. Silverman, P.M. Tolstoy, G.S. Denisov, H.-H. Limbach
“NMR Studies of Active Site Properties of Human Carbonic Anhydrase II using 15N labeled 4-Methylimidazole as a Local Probe and Histidine Hydrogen Bond Correlations”
Chem. Eur. J., 2014, accepted
DOI: 10.1002/chem.201404083
By using a combination of liquid and solid-state NMR spectroscopy, 15N-labeled 4-methylimidazole (4-MI) as a local probe of the environment has been studied: 1) in the polar, wet Freon CDF3/CDF2Cl down to 130 K, 2) in water at pH 12, and 3) in solid samples of the mutant H64A of human carbonic anhydrase II (HCA II). In the latter, the active-site His64 residue is replaced by alanine; the catalytic activity is, however, rescued by the presence of 4-MI. For the Freon solution, it is demonstrated that addition of water molecules not only catalyzes proton tautomerism but also lifts its quasidegeneracy. The possible hydrogen-bond clusters formed and the mechanism of the automerism are discussed. Information about the imidazole hydrogen-bond geometries is obtained by establishing a correlation between published 1H and 15N chemical shifts of the imidazole rings of histidines in proteins. This correlation is useful to distinguish histidines embedded in the interior of proteins and those at the surface, embedded in water. Moreover, evidence is obtained that the hydrogen-bond geometries of His64 in the active site of HCA II and of 4-MI in H64A HCA II are similar. Finally, the degeneracy of the rapid tautomerism of the neutral imidazole ring His64 reported by Shimahara et al. (J. Biol. Chem. 2007, 282, 9646) can be explained with a wet, polar, nonaqueous active-site conformation in the inward conformation, similar to the properties of 4-MI in the Freon solution. The biological implications for the enzyme mechanism are discussed.
Школа для молодых ученых
Inorganic Chemistry 2014
A. Miroslavov, Y. Polotskii, V. Gurzhiy, A. Ivanov, A. Lumpov, M. Tyupina, G. Sidorenko, P. Tolstoy, D. Maltsev, D. Suglobov
“Technetium and Rhenium Pentacarbonyl Complexes with C2 and C11 ω-Isocyanocarboxylic Acid Esters”
Inorg. Chem. 2014, Article ASAP
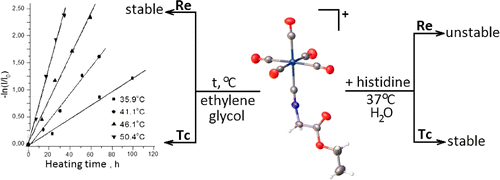
Technetium(I) and rhenium(I) pentacarbonyl complexes with ethyl 2-isocyanoacetate and methyl 11-isocyanoundecanoate, [M(CO)5(CNCH2COOEt)]ClO4 (M = Tc (1) and Re (2)) and [M(CO)5(CN(CH2)10COOMe)]ClO4 (M = Tc (3) and Re (4)), were prepared and characterized by IR, 1H NMR, and 13C{1H} NMR spectroscopy. The crystal structures of 1 and 2 were determined using single-crystal X-ray diffraction. The kinetics of thermal decarbonylation of technetium complexes 1 and 3 in ethylene glycol was studied by IR spectroscopy. The rate constants and activation parameters of this reaction were determined and compared with those for [Tc(CO)6]+. It was found that rhenium complexes 2 and 4 were stable with respect to thermal decarbonylation. Histidine challenge reaction of complexes 1 and 2 in phosphate buffer was examined by IR spectroscopy. In the presence of histidine, the rhenium pentacarbonyl isocyanide complex partially decomposes to form an unidentified yellow precipitate. Technetium analogue 1 is more stable under these conditions.
Делегация Высшей медицинской школы Ганновера
РЦ посетила делегация Высшей медицинской школы Ганновера:
- Dr. Tilman Fabian, Management Cluster of Excellence “Rebirth”, expert in management of interdisciplinary research projects
- Prof. Dr. Ullrich Martin, Leibniz Research Laboratory for Biotechnology and Artificial Organs, expert in iPS cells
- Prof. Dr. Denise Hilfiker-Kleiner, Dean of Research, Hannover Medical School, expert in experimental Cardiology
- Dr. Robert Zweigerdt, Leibniz Research Laboratory for Biotechnology and Artificial Organs, expert in cell mass production/bioreactors
- Dr. Andres Hilfiker, Leibniz Research Laboratory for Biotechnology and Artificial Organs, expert in Tissue Engineering
- Prof. Dr. Axel Schambach, PhD, Department of Hematology, Hannover Medical School, expert in Experimental Hematology
- Prof. Dr. Alexander Heisterkamp, Leibniz University Hannover, Hannover Laser Center, expert in Biophotonics