Gurinov, A. A.; Mauder, D.; Akcakayiran, D.; Findenegg, G. H.; Shenderovich, I. G.
“Does Water Affect the Acidity of Surfaces? The Proton-Donating Ability of Silanol and Carboxylic Acid Groups at Mesoporous Silica.”
ChemPhysChem 2012, 13 (9), 2282-2285.
DOI: 10.1002/cphc.201200204
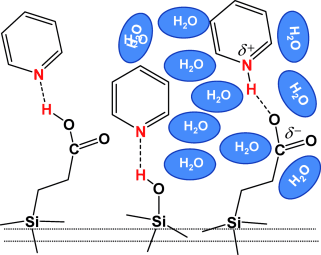
Solvation at the interphase:
A study of the influence of water on the effective acidity of silanol and carboxylic acid groups of propionic acid functionalized SBA-15 reveals that to affect the proton-donating ability of an acidic group at the surface, water should be able to form a solvation shell around that group. As a result, water does not affect the acidity of native SBA-15 but dramatically enhances that of SBA-15 functionalized with propionic acid moieties.






