M. A. Kinzhalov , A. S. Legkodukh, T. B. Anisimova, A. S. Novikov, V. V. Suslonov, K. V. Luzyanin , V. Yu. Kukushkin
“Tetrazol-5-ylidene Gold(III) Complexes frоm Sequential [2 + 3] Cycloaddition of Azide to Metal-Bound Isocyanides and N4 Alkylation”
Organometallics, 2017, 36, 3974–3980
DOI: 10.1021/acs.organomet.7b00591
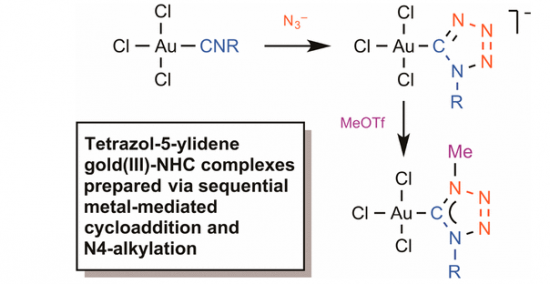
The reaction between equimolar amounts of the isocyanide complexes [AuCl3(CNR)] [R = 2,6-Me2C6H3 (Xyl), 1a; 2,4,6-Me3C6H2 (Mes), 1b; Cy, 1c; t-Bu, 1d] and tetrabutylammonium azide (2) proceeds in CH2Cl2 at room temperature for ∼10 min to furnish the gold(III) tetrazolates [n-Bu4N][AuCl3(CN4R)] (3a–d), which were obtained in 89–95% yields after purification. Subsequent reaction between equimolar amounts of 3a–d and methyl trifluoromethanesulfonate (MeOTf) proceeds in CH2Cl2 at −70 °C for ∼30 min to give the corresponding gold(III) complexes [AuCl3(CaN(Me)N2NbR)]a–b (5a–d) bearing 1,4-disubstituted tetrazol-5-ylidene ligands (69–75%). Complexes 3a–d were obtained as pale-yellow solids and characterized by elemental analyses (C, H, N), HRESI–-MS, FTIR, and 1H and 13C{1H} NMR spectroscopies. Complexes 5a–d were obtained as colorless solids and characterized by elemental analyses (C, H, N), HRESI+-MS, and 1D (1H and 13C{1H}) and 2D (1H,13C-HMBC) NMR spectroscopies. In addition, the structures of 3a, 3b, 3c, and 5a were established by single-crystal X-ray diffraction. Analysis of the Wiberg bond indices (WI) for gas phase-optimized model structures of 3a–c and 5a computed using the natural bond orbital (NBO) partitioning scheme disclosed a higher degree of electron density delocalization in the CN4 moiety of carbene 5a when compared to tetrazolate 3a–c. Results of DFT calculations for a model system reveal that the mechanism for the cycloaddition of an azide to the isocyanide ligand in [AuCl3(CNMe)] is stepwise and involves nucleophilic attack of N3– on the N atom of CNMe followed by ring closure. The addition is both kinetically and thermodynamically favorable and occurs via the formation of an acyclic NNNCN intermediate, whereas the cyclization is the rate-determining step.