 Ранее, 22.06.2016, мы приводили объективные доказательства существования головного мозга у директора РЦ МРМИ. Сегодня мы спешим поделиться новой информацией: директор не бесхребетный (см. картинку). Анализ приведенного изображения трактуется специалистами как признаки существования у пациента позвоночника, что позволило классифицировать его как представителя типа Chordata. В дальнейшем коллектив центра постарается установить, откуда у директора растут руки.
Ранее, 22.06.2016, мы приводили объективные доказательства существования головного мозга у директора РЦ МРМИ. Сегодня мы спешим поделиться новой информацией: директор не бесхребетный (см. картинку). Анализ приведенного изображения трактуется специалистами как признаки существования у пациента позвоночника, что позволило классифицировать его как представителя типа Chordata. В дальнейшем коллектив центра постарается установить, откуда у директора растут руки.
Хордовые
Апрель
В Апреле выполнено 2877 заявок на сервисные измерения.
Измерено:
- 2729 спектр 1H
- 421 спектра 13C
- 130 спектров DEPT
- 69 спектров COSY
- 53 спектра NOESY
- 49 спектра 31Р
- 115 спектров 19F
Выполнено 323 заявок на исследовательскую работу.
Microporous and Mesoporous Materials, 2018, 132-142
E.A. Krylova, M.G. Shelyapina, P. Nowak, H. Harańczyk, M. Chislov, I.A. Zvereva, A.F. Privalov, M. Becher, M. Vogel, V. Petranovskii
“Mobility of water molecules in sodium- and copper-exchanged mordenites: Thermal analysis and 1H NMR study”
Microporous and Mesoporous Materials, 2018, 265, 132-142
DOI:10.1016/j.micromeso.2018.02.010
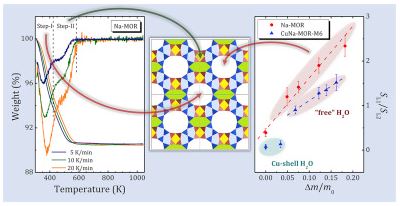
Comprehensive research of water behavior in Na- and Cu-mordenites with different Na/Cu ratio was done. Several steps of dehydration process were detected and analyzed, taking into account difference in chemical composition of the samples, reaction models and corresponding kinetic equations. Activation energies for these steps were calculated. It was shown that the majority of dehydration steps for all zeolite samples studied might be associated with chemical reaction mechanism corresponding to the second order kinetic model, except for the most high-temperature step for Cu-mordenite, for which the third-order model has the higher correlation coefficient. A detailed analysis of rehydration processes was studied by proton NMR spectroscopy. The obtained results allow one to distinguish different types of water and to associate them with a certain localization of water molecules in zeolite voids: the main channel for both Na and Cu-mordenites; a side pocket of Na-mordenite; molecules coordinated with Cu2+ cations in Cu-mordenite. The diffusion measurements carried out using static field gradient NMR technique proved that the water diffusion character below 300 K is essentially intracrystalline, whereas above 300 K it becomes intercrystalline. The activation energy of intercrystalline diffusion is about 28 kJ/mol and does not depend on the Na/Cu ratio. That allows us to suppose that in the studied zeolites the intercrystalline diffusion is governed by the morphology of the sample mainly.
Microporous and Mesoporous Materials, 2018, 220-228
Y.M. Zhukov, M.G. Shelyapina, I.A. Zvereva, A.Y. Efimov, V. Petranovskii
“Microwave assisted versus convention Cu2+ exchange in mordenite”
Microporous and Mesoporous Materials, 2018, 259, 220-228
DOI:10.1016/j.micromeso.2017.10.013
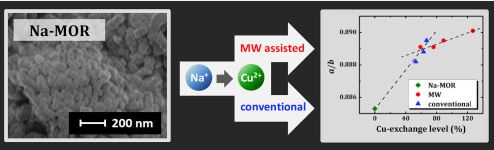
Sodium mordenite was Cu2+-exchanged by conventional methods at ambient temperature (accepted as 20 °C) and with microwave radiation at 100 ± 1 °C. To increase the copper content, we repeat the exchange procedure several times. Both the degree of Cu2+ exchange and the environment of the Cu2+ ions depend on the method of exchange. Neither conventional, nor microwave methods do not destruct the topology of mordenite framework. XRD pattern of the mordenite persists, but slight elastic deformation and some dealumination of the surface layer occurs. For all the studied samples, all the copper ions are in Cu2+ state, neither Cu1+ nor Cu0 were detected. All the copper ions play the role of charge-balancing counter-cations. Their placement into the interior of zeolite causes the channel contraction due to electrostatic interactions of double charged cations with [AlO4]δ– units. The increase of copper content is accompanied by increasing of number of water molecules per unit cell. In fully hydrated samples the copper cations are effectively separated from the zeolite framework and are fully surrounded by a water ligand shell.
Конференция: Современное развитие магнитного резонанса
C 24 по 28 сентября 2018 г. (Казань, Россия) пройдет очередная конференция «Modern Development of Magnetic Resonance 2018»
Org. Chem. Front., 2018, 226-231
M. S. Ledovskaya, K. S. Rodygina, V. P. Ananikov
“Calcium-mediated one-pot preparation of isoxazoles with deuterium incorporation”
Org. Chem. Front., 2018, 5, 226-231
DOI:10.1039/C7QO00705A
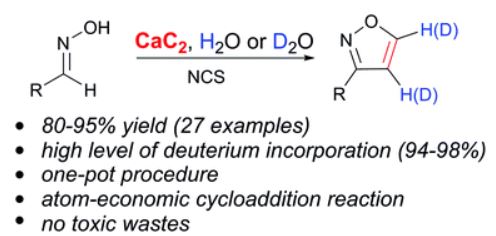
In this work, a novel synthetic methodology for the one-pot preparation of isoxazoles directly from the reaction of calcium carbide with aldoximes is reported. Calcium carbide acts as a safe and inexpensive acetylene source and, in addition, as a source of the Ca(OH)2 base to enable the generation of nitrile oxide. Various 3-substituted isoxazoles were synthesized from the corresponding aldoximes in good yields (up to 95%) and a series of new deuterated 4,5-dideuteroisoxazoles were prepared.
Школа-конференция
С 15 по 20 сентября 2018 г в Санкт-Петербурге пройдет V международная школа-конференция для молодых ученых «Магнитный резонанс и магнитные явления в химии и биофизике». О конференции можно прочитать тут.
Int. J. Nanomedicine, 2018, 1471-1482
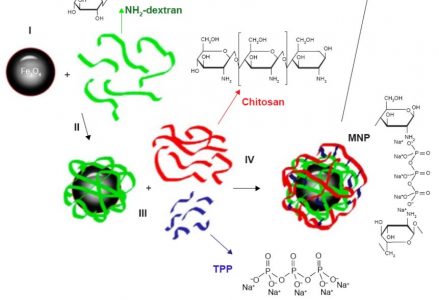
M. Shevtsov, B. Nikolaev, Y. Marchenko, L. Yakovleva, N.V. Skvorzov, A. Mazur, P. Tolstoy, V. Ryzhov, G. Multhoff
“Targeting experimental orthotopic glioblastoma with chitosan-based superparamagnetic iron oxide nanoparticles (CS-DX-SPIONs)”
Int. J. Nanomedicine, 2018, 13, 1471-1482
DOI: 10.2147/IJN.S152461
Glioblastoma is the most devastating primary brain tumor of the central nervous system in adults. Magnetic nanocarriers may help not only for a targeted delivery of chemotherapeutic agents into the tumor site but also provide contrast enhancing properties for diagnostics using magnetic resonance imaging (MRI)
ACS Biomater. Sci. Eng, 2018, 491-501
M. Promzeleva, T.V. Volkova, A.N. Proshin, O.I. Siluykov, A. Mazur, P.M. Tolstoy, S.P. Ivanov, F. Kamilov, I.V. Terekhova
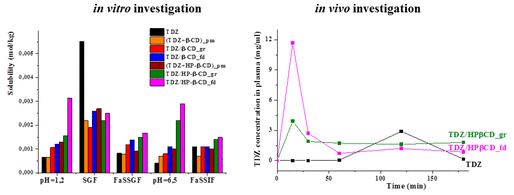
“Improved biopharmaceutical properties of oral formulations of 1,2,4-thiadiazole derivative with cyclodextrins: in vitro and in vivo evaluation”
ACS Biomater. Sci. Eng, 2018, 4(2), 491-501
DOI: 10.1021/acsbiomaterials.7b00887
The synthesized 1,2,4-thiadiazole derivative displaying biological activity has low aqueous solubility and dissolution rate. Novel oral formulations of thiadiazole with β- and hydroxypropyl-β-cyclodextrins were obtained by grinding and freeze-drying methods with the purpose to improve the aqueous solubility. Complex formation of 1,2,4-thiadiazole derivative with cyclodextrins was confirmed by means of solid-state 13C MAS CP/TOSS NMR. Solubility, dissolution rate and permeability of the solid inclusion complexes were evaluated in different biorelevant media (SGF, FaSSGF, FaSSIF) simulating the conditions in the gastrointestinal tract. It was demonstrated that the content of biorelevant media affects the properties of the inclusion complexes. In particular, solubilizing effect of cyclodextrins became less pronounced when the micelles of taurocholic acid and lecithin are formed in the dissolution media. The inclusion of thiadiazole into cyclodextrin cavity is in competition with its partitioning into the micelles and this should be taken into account when the in vivo behavior is predicted. The results of in vitro and in vivo experiments were found to be in agreement and showed the highest solubility, dissolution rate and bioavailability of the freeze-dried complexes of thiadiazole with hydroxypropyl-β-cyclodextrin. These complexes can be proposed as more effective dosage forms for oral administration.
J. Phys. Chem. C, 2018, 1711-1720
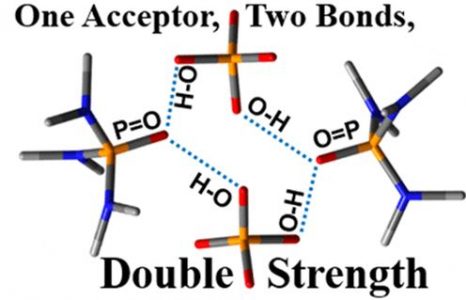
E.Yu. Tupikina, M. Bodensteiner, P.M. Tolstoy, G.S. Denisov, I.G. Shenderovich
“P=O Moiety as an Ambidextrous Hydrogen Bond Acceptor”
J. Phys. Chem. C, 2018, 122(3), 1711-1720
DOI: 10.1021/acs.jpcc.7b11299
Hydrogen bond patterns of crystals of phosphinic, phosphonic, and phosphoric acids and their cocrystals with phosphine oxides were studied using 31P NMR and single-crystal X-ray diffraction. Two main factors govern these patterns and favor or prevent the formation of cocrystals. The first one is a high proton-accepting ability of the P═O moiety in these acids. As a result, this moiety effectively competes with other proton acceptors for hydrogen bonding. For example, this moiety is a stronger proton acceptor than the C═O moiety of carboxylic acids. The second factor is the inclination of the P═O moiety of both the acids and the oxides to form two hydrogen bonds at once. The peculiarity of these bonds is that they weaken each other to a little degree only. In order to highlight this point, we are using the term “ambidextrous”. These two features should govern the interactions of P═O moiety with water and other proton donors and acceptors in molecular clusters, the active sites of enzymes, soft matter, and at surfaces.