Дж.Н. Коншина, А.В. Данилова, З.А. Темердашев, С.Н. Болотин, А.А. Гуринов, В.В. Коншин
“Получение и некоторые свойства силикагеля с иммобилизованной формазановой группой”
Журнал прикладной химии, 2016, 89(4), 476-483
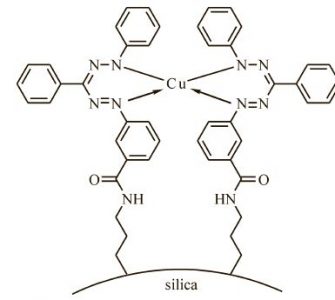
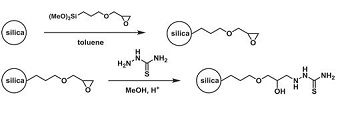
Предложен и охарактеризован новый сорбционный материал — силикагель с ковалентно иммобилизованной формазановой группой, получаемый сочетанием иммобилизованной соли арилдиазония с фенилгидразоном бензальдегида. Определены некоторые равновесные и кинетические характеристики сорбции Cu(II), Co(II), Ni(II) и Cd(II) из растворов полученным модифицированным силикагелем, показана его эффективность для концентрирования Cu(II) из растворов сложного состава. По данным ЭПР определено координационное окружение Cu(II) в комплексе на поверхности сорбента.