В июле выполнено 1725 заявок на сервисные измерения.
Измерено:
- 1611 спектров 1H
- 317 спектров 13C
- 70 спектров DEPT
- 70 спектров COSY
- 24 спектра NOESY
- 47 спектров 31Р
- 54 спектра 19F
Выполнено 253 заявки на исследовательскую работу.
В июле выполнено 1725 заявок на сервисные измерения.
Измерено:
Выполнено 253 заявки на исследовательскую работу.
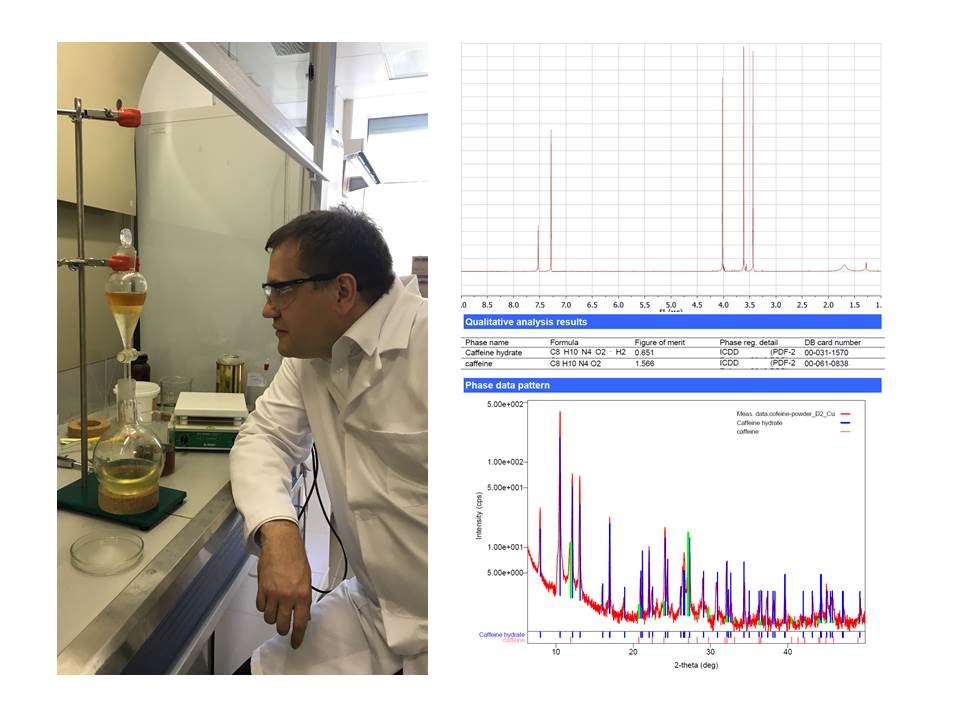
В связи с большим количеством поступающих в РЦ заявок, в целях увеличения бодрости и эффективности работы сотрудников в центре налажено получение кофеина из чайного листа. Спектр 1H ЯМР и порошковая рентгенограмма, зарегистрированная в РЦ РДМИ, подтвердили чистоту выделенного соединения. Ожидаемый прирост продуктивности оказался полностью скомпенсирован затратами времени на получение кофеина. Ведутся дополнительные разработки.
В июне выполнено 1912 заявок на сервисные измерения.
Измерено:
Выполнено 295 заявок на исследовательскую работу.

К коллективу РЦ МРМИ присоединилась новая сотрудница: Ирина Лушпинская будет заниматься твердотельной спектроскопией ЯМР, магнитно-резонансной томографией и спектроскопией ЯКР.
В мае выполнено 2438 заявок на сервисные измерения.
Измерено:
Выполнено 324 заявки на исследовательскую работу.
A. S. Mikherdov , A. S. Novikov , M. A. Kinzhalov , V. P. Boyarskiy, G. L. Starova, A. Yu. Ivanov, V. Yu. Kukushkin
“Halides Held by Bifurcated Chalcogen-Hydrogen Bonds. Effect of µ(S,N-H)Cl Contacts on Dimerization of Cl(carbene)PdII Species ”
Inorg. Chem. , 2018, 57(6), 3420-3433
DOI:10.1021/acs.inorgchem.8b00190
The reaction of cis-[PdCl2(CNCy)2] (1) with thiazol-2-amines (2–10) leads to the C,N-chelated diaminocarbene-like complexes [PdCl{C(N(H)4,5-R2-thiazol-2-yl)NHCy}(CNCy)] (11–14; 82–91%) in the case of 4,5-R2-thiazol-2-amines (R, R = H, H (2), Me, Me (3), −(CH2)4– (4)) and benzothiazol-2-amine (5) or gives the diaminocarbene species cis-[PdCl2{C(N(H)Cy)N(H)4-R-thiazol-2-yl}(CNCy)] (15–19; 73–93%) for the reaction with 4-aryl-substituted thiazol-2-amines (R = Ph (6), 4-MeC6H4 (7), 4-FC6H4 (8), 4-ClC6H4 (9), 3,4-F2C6H3 (10)). Inspection of the single-crystal X-ray diffraction data for 15–17 and 19 suggests that the structures of all these species exhibit previously unrecognized bifurcated chalcogen–hydrogen bonding μ(S,N–H)Cl and also PdII···PdII metallophilic interactions. These noncovalent interactions collectively connect two symmetrically located molecules of 15–17 and 19, resulting in their solid-state dimerization. The existence of the μ(S,N–H)Cl system and its strength (6–9 kcal/mol) were additionally verified/estimated by a Hirshfeld surface analysis and DFT calculations combined with a topological analysis of the electron density distribution within the formalism of Bader’s theory (AIM method) and NBO analysis. The observed noncovalent interactions are jointly responsible for the dimerization of 15–19 not only in the solid phase but also in CHCl3 solutions, as predicted theoretically by DFT calculations and confirmed experimentally by FTIR, HRESI-MS, 1H NMR, and diffusion coefficient NMR measurements. Available CCDC data were processed under the new moiety angle, and the observed μ(S,E–H)Cl systems were classified accordingly to E (E = N, O, C) type atoms.
 Иногда мы задаемся вопросом, сколько запросов поступает от пользователей рц МРМИ. Коллектив рц МРМИ в апреле добрался до юбилейной 100000 заявки попавшей к нам от пользователя рц Калинина С.А.
Иногда мы задаемся вопросом, сколько запросов поступает от пользователей рц МРМИ. Коллектив рц МРМИ в апреле добрался до юбилейной 100000 заявки попавшей к нам от пользователя рц Калинина С.А.
 РЦ «Магнитно-резонансные методы исследования» приглашает на работу (1.0 ставки) специалиста по спектроскопии ЯМР растворов
РЦ «Магнитно-резонансные методы исследования» приглашает на работу (1.0 ставки) специалиста по спектроскопии ЯМР растворов
Стартовая зарплата (оклад + ОЦО + премия) 30 т.р. до вычета налогов.
Условия работы
— 1.0 ставки (40 часов в неделю), начало работы возможно с 1.06.2018
— основное место работы (без возможности совместительства)
— работа в комфортных условиях на современном оборудовании «Bruker»
Обязанности сотрудника
‐ выполнение заявок пользователей (своих исследований нет)
‐ поддержка новостей и актуальной информации на сайте ресурсного центра
Прием на работу по результатам собеседования. Желателен опыт работы.
По всем вопросам обращайтесь к директору РЦ Толстому Петру Михайловичу
peter.tolstoy@spbu.ru, +7 921 430‐81‐91
A. V.Protas, E.A.Popova, O. V.Mikolaichuk, Y. B.Porozov, A. R.Mehtiev, I. Ott, G. V. Alekseev, N. A.Kasyanenko, R. E.Trifonov
“Synthesis, DNA and BSA binding of Pd(II) and Pt(II) complexes featuring tetrazolylacetic acids and their esters”
Inorganica Chimica Acta, 2018, 473, 133-144
DOI:10.1016/j.ica.2017.12.040
Two series of palladium(II) and platinum(II) complexes featuring esters of tetrazol-1-yl and tetrazol-5-ylacetic acids {trans-[PdCl2L2] and trans-[PtCl2L2], L = 5-methyl-1H-tetrazol-1-ylacetic acid and its ethyl, butyl, isobutyl esters (1–5); 2-R-2H-tetrazol-5-ylacetic acid and its ethyl esters, R = tBu, CH2CH2OH (6–10)} were synthesized and their binding to calf-thymus DNA (CT DNA) and bovine serum albumin (BSA) were studied by means of experimental (CD, UV, viscometry, fluorometric and electrophoretic techniques) and theoretical methods. According to the spectrophotometric data, the interaction of the metal complexes with CT DNA is observed. The significant increase of melting point of CT DNA in the presence of the metal complexes (ΔTm = 8–13 °C) indicates strong stabilization of the DNA helix. Electrophoretic studies demonstrate the ability of the metal complexes to interact with pBR322 plasmid DNA and to change its mobility. According to the data of the fluorescence quenching technique, binding with constants (Kbin) of Pd(II) complexes with BSA are in the range 0.83–4.12 × 105 L M−1. The molecular docking studies show the minor groove binding behavior of tetrazole-containing palladium(II) and platinum(II) complexes to DNA (ΔGbinding. −5.56 − −6.12 kcal/mol) and effective binding to BSA via the favored binding site Trp213 (ΔGbinding −7.2 − −7.56 kcal/mol). The complex trans-[PtCl2(2-tert-butyl-tetrazol-5-ylacetic acid)2] exhibited noticeable antiproliferative activity in two human cancer cell lines with IC50 values of 11.40 µM in HT-29 cells and 11.02 µM in MDA-MB-231 cell line.
A. S. Mikherdov, A. S. Novikov, M. A. Kinzhalov, A. A. Zolotarev, V. P. Boyarskiy
“Intra-/Intermolecular Bifurcated Chalcogen Bonding in Crystal Structure of Thiazole/Thiadiazole Derived Binuclear (Diaminocarbene)PdII Complexes”
Crystals, 2018, 8(3), 112
DOI:10.3390/cryst8030112
The coupling of cis-[PdCl2(CNXyl)2] (Xyl = 2,6-Me2C6H3) with 4-phenylthiazol-2-amine in molar ratio 2:3 at RT in CH2Cl2 leads to binuclear (diaminocarbene)PdII complex 3c. The complex was characterized by HRESI+-MS, 1H NMR spectroscopy, and its structure was elucidated by single-crystal XRD. Inspection of the XRD data for 3c and for three relevant earlier obtained thiazole/thiadiazole derived binuclear diaminocarbene complexes (3a EYOVIZ; 3b: EYOWAS; 3d: EYOVOF) suggests that the structures of all these species exhibit intra-/intermolecular bifurcated chalcogen bonding (BCB). The obtained data indicate the presence of intramolecular S•••Cl chalcogen bonds in all of the structures, whereas varying of substituent in the 4th and 5th positions of the thiazaheterocyclic fragment leads to changes of the intermolecular chalcogen bonding type, viz. S•••π in 3a,b, S•••S in 3c, and S•••O in 3d. At the same time, the change of heterocyclic system (from 1,3-thiazole to 1,3,4-thiadiazole) does not affect the pattern of non-covalent interactions. Presence of such intermolecular chalcogen bonding leads to the formation of one-dimensional (1D) polymeric chains (for 3a,b), dimeric associates (for 3c), or the fixation of an acetone molecule in the hollow between two diaminocarbene complexes (for 3d) in the solid state. The Hirshfeld surface analysis for the studied X-ray structures estimated the contributions of intermolecular chalcogen bonds in crystal packing of 3a–d: S•••π (3a: 2.4%; 3b: 2.4%), S•••S (3c: less 1%), S•••O (3d: less 1%). The additionally performed DFT calculations, followed by the topological analysis of the electron density distribution within the framework of Bader’s theory (AIM method), confirm the presence of intra-/intermolecular BCB S•••Cl/S•••S in dimer of 3c taken as a model system (solid state geometry). The AIM analysis demonstrates the presence of appropriate bond critical points for these interactions and defines their strength from 0.9 to 2.8 kcal/mol indicating their attractive nature