M. Chizhova, D. Dar’in, M. Krasavin
“Complications in the Castagnoli-Cushman reaction: An unusual course of reaction between cyclic anhydrides and sterically hindered indolenines”
Tetrahedron Letters, 2017, 58(35), 3470-3473
DOI: 10.1016/j.tetlet.2017.07.077
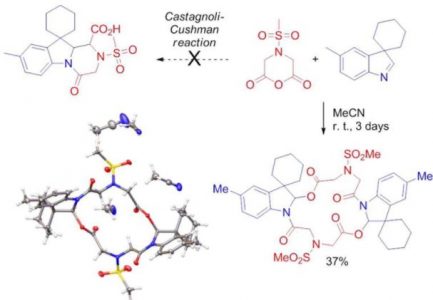
Attempted reaction of indolenines (which represent rather sterically hindered cyclic imines) with a series of dicarboxylic acid anhydrides yielded no expected product, the Castagnoli-Cushman lactam. Instead, products presumably formed via N-acyliminium species trapping by a carboxylate anion. Among them, hydrolytically labile 2:2 adducts of an indolenine and a cyclic anhydride, containing a 16-membered cyclic core, are particularly intriguing. This result contradicts the recently reported successful Castagnoli-Cushman reaction of indolenines with homophthalic anhydride suggesting a mechanistic switch in the course of the reaction.