P.A. Sakharov, N.V. Rostovskii, A.F. Khlebnikov, M.S. Novikov
“Annulation of five-membered cyclic enols with 3-aryl-2H-azirines: Catalytic versus non-catalytic cycloaddition”
Tetrahedron, 2017, 73(31), 4663-4670.
DOI: 10.1016/j.tet.2017.06.037
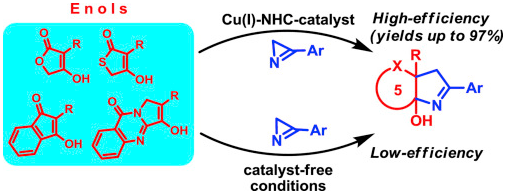
A copper(I)-NHC-catalyzed annulation of 5-membered cyclic enols of furan, thiophene and indene family with 3-aryl-2H-azirines has been developed to provide a rapid access to furo[3,4-b]pyrrole, thieno[3,4-b]pyrrole, indeno[1,2-b]pyrrole derivatives. The reaction proceeds via a copper-initiated N-C2 azirine bond cleavage with the retention of the C=N double bond in the annulation product. The reaction of tetronic acids with 3-aryl-2H-azirines can also proceed under catalyst-free conditions, through a double addition of the enol to the azirine, but this route provides poor yields of the annulation products. This is the first example of the N-C2 bond cleavage in 2-unsubstituted 2H-azirines in the absence of a transition metal catalyst.