Обновленная версия Рекламной брошюры РЦ МРМИ

Обновленная версия Рекламной брошюры РЦ МРМИ

Михаил Вовк провел экскурсию-лекцию для учеников старшей школы. Нашим посетителям была предложена вводная лекция по основам ЯМР и истории развития данного метода, приведены примеры применения спектроскопии ЯМР для иследования структуры органических молекул, металлоорганических комплексов, белков. Продемонстрировано оборудование для проведения микротомографии и спектроскопии ЯМР твердотельных объектов.
M.A. Kuznetsov, A.N. Shestakov, M. Zibinsky, M. Krasavin, C.T. Supuran, S. Kalinin, Muhammet Tanç
“Synthesis, structure and properties of N-aminosaccharin — A selective inhibitor of human carbonic anhydrase I”
Tetrahedron Lett., 2017, 58, 172-174
DOI:10.1016/j.tetlet.2016.12.005
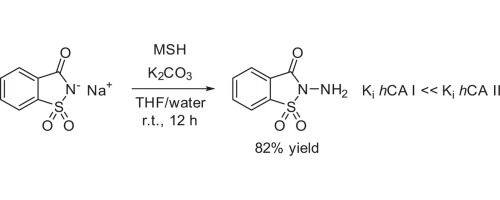
Previously unknown N-aminosaccharin was prepared in good yield via the one-step direct amination of saccharin sodium salt with hydroxylamine-O-mesitylenesulfonic acid (MSH) and its reactivity investigated. N-aminosaccharin and its derivatives were tested against hCA isoforms and the parent compound was identified to be a selective, low micromolar inhibitor (Ki = 8.8 μM) of hCA I. These findings provide a ligand-efficient starting point for the design of potent hCA I inhibitors – a promising drug target for retinal/cerebral edema treatment.
A.N. Shestakov, A.S. Pankova, P.Golubev, A.F. Khlebnikov, M.A. Kuznetsov
“Brønsted acid mediated cyclizations of ortho-aryl(ethynyl)pyrimidines”
Tetrahedron, 2017, 73, 3939-3948
DOI:10.1016/j.tet.2017.05.070
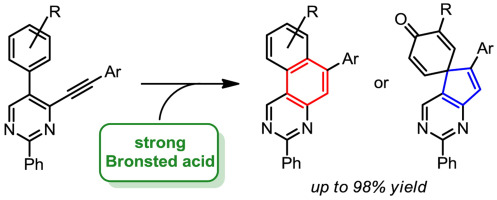
A high-yielding procedure for the synthesis of 5-aryl-4-(arylethynyl)pyrimidines from easily available 2-aryl-3-hydroxyacrylates is reported. These pyrimidines readily undergo cyclization in strong Brønsted acids and, depending on the substitution in alkynylpyrimidines and the water content of the reaction mixture, yield either benzo[f]quinazolines or derivatives of spiro[cyclohexa-2,5-diene-1,5′-cyclopenta[d]pyrimidin]-4-one. In most cases the cyclization proceeds nearly quantitatively. DFT calculations support the proposed mechanisms induced by the protonation of the triple bond in 5-aryl-4-(arylethynyl)pyrimidines. Fluorescent properties of the obtained heterocycles are also described.