M.S. Mishina, A.Yu. Ivanov, P.S. Lobanov, D.V. Dar’in
“A New Synthesis of 2-Aminoindoles and 6-Aminopyrrolo[3,2-d]pyrimidines from π-Deficient 1,2-Dihaloarenes and Geminal Enediamines”
Synthesis, 2016, 48, 2851-2862
DOI:10.1055/s-0035-1561645
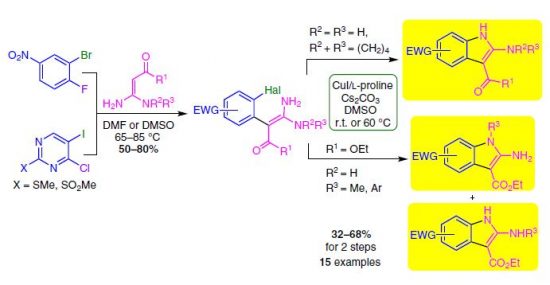
An efficient approach for the synthesis of fused 2-aminopyrroles via geminal enediamines and π-deficient 1,2-dihaloarenes is presented. The two-step methodology includes aromatic nucleophilic substitution of the activated halogen of dihaloarene with enediamine C-nucleophilic center followed by Cu-catalyzed intramolecular N-arylation. This approach allows access to a variety of 2-amino-6-nitroindoles and 6-aminopyrrolo[3,2-d]pyrimidines (including N-mono- and N,N-disubstituted) in moderate and good yields under mild conditions.