Archive for 30.12.2015
Tetrahedron Lett., 2015 (56) 2200-2202
D.S. Ryabukhin, A.V. Vasilyev
“A synthesis of 4-aryl quinolin-2(1H)-ones by acidic zeolite promoted intramolecular cyclization of N-aryl amides of 3-arylpropynoic acids”
Tetrahedron Lett., 2015, 56, 2200-2202
DOI:10.1016/j.tetlet.2015.03.060
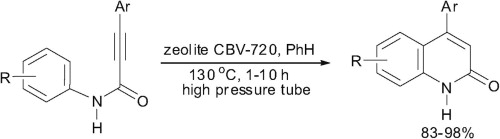
N-Aryl amides of 3-arylpropynoic acids are intramolecularly cyclized into 4-aryl quinolin-2(1H)-ones in high yields without any by-products under the action of acidic zeolite CBV-720 in benzene at 130 °C in a glass high pressure tube.
Что делать с ненужным FID?
Уважаемые пользователи, если полученные после преобразования Фурье спектры вас не радуют, можно преобразовать их в елку!
Заливка Гелия

Сегодня в РЦ прошли плановые работы по заливке гелия.
Предновогодние образцы

Спасибо нашим пользователям за создание новогоднего антуража!
Tetrahedron, 2015 (71) 1940-1951
A.V. Galenko, A.F. Khlebnikov, M.S. Novikov, M.S. Avdontseva
“Synthesis of 3-(1,2-dioxoethyl)- and 2,3-dicarbonyl-containing pyrroles”
Tetrahedron, 2015, 71, 1940-1951
DOI:10.1016/j.tet.2015.02.030
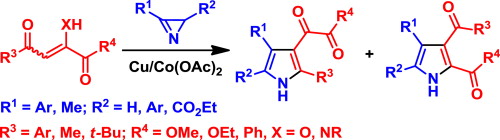
The transition metal-catalyzed reaction of 2H-azirines with 1,2,4-tricarbonyl compounds leads to 3-(1,2-dioxoethyl)- and 2,3-dicarbonyl-pyrrole derivatives, useful building blocks for the preparation of 3-heterocyclyl pyrroles and pyrroles fused with heterocycles. The influence of catalysts and the reaction conditions on the yields of pyrroles and the regioselectivity of the reaction were studied. Experimental and theoretical results suggest that the reaction proceeds via the formation of an intermediate azirine–metal complex and subsequent nucleophilic N–C3 bond cleavage.
Гости РЦ, Китай (Чжэнчжоу)

РЦ посетили гости из Центра образования русского языка Международного института образования (Чжэнчжоуский университет, Китай). Чжоу То и его коллеги выразили интерес к межвузовскому сотрудничеству по направлению химия и материаловедение и познакомились с организацией и принципами работы РЦ МРМИ.
Экскурсия для физиков

Михаил Вовк проводит обзорную экскурсию в РЦ для студентов физиков первого года обучения.
Берлин, семинар
П.М. Толстой прочел доклад «SPbU Research Park, Center for Magnetic Resonance: possibilities of joint research projects» на семинаре Berlin — St. Petersburg Workshop on Structure and Dynamics of Nanoscopic Matter, прошедшем в Берлине 10-11.12.15.
J. Org. Chem. 2015, 80, 5546-5555
N.A. Danilkina, A.G. Lyapunova, A.F. Khlebnikov, G.L. Starova, S. Brase, I.A. Balova
“Ring-Closing Metathesis of Co2(CO)6–Alkyne Complexes for the Synthesis of 11-Membered Dienediynes: Overcoming Thermodynamic Barriers”
J. Org. Chem., 2015, 80, 5546-5555
DOI:10.1021/acs.joc.5b00409
The feasibility of ring-closing metathesis (RCM) as a synthetic entry to 10- and 11-membered dienediynes fused to a benzothiophene core was explored by experimental and theoretical investigations. An established sequence of iodocyclization of o-(buta-1,3-diynyl)thioanisoles followed by Sonogashira coupling to form diethynylbenzothiophenes was used to synthesize terminal benzothiophene-fused enediyne diolefins as substrates for RCM. Encountering an unexpected lack of reactivity of these substrates under standard RCM conditions, we applied DFT calculations to reveal that the underlying cause was a positive change in Gibbs free energy. The change in Gibbs free energy was also found to be positive for RCM of indole- and benzannulated terminal diolefins when affording smaller than 12-membered rings. We found that modification of the enediyne–diolefin substrate as the Co2(CO)6–alkyne complex allowed the target benzothiophene-fused 11-membered dienediyne to be obtained via RCM; the alkyne complexation strategy therefore provides one valid technique for overcoming challenges to macrocyclization of this kind.

