A. S. Antonov, A. F. Pozharskii, V. A. Ozeryanskii, A. Filarowski,
K. Yu. Suponitsky, P. M. Tolstoy, M. A. Vovk
“Ring Lithiation of 1,8-Bis(dimethylamino)naphthalene: Another Side of the ‘Proton Sponge Coin’”
Dalton Trans., 2015, accepted
DOI:10.1039/C5DT02482J
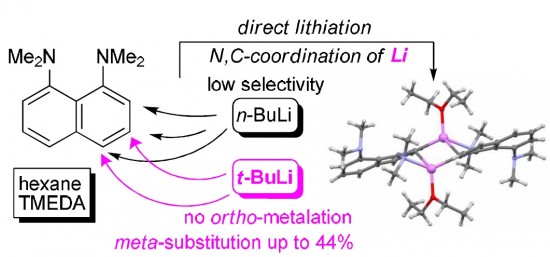
It has been found that 1,8-bis(dimethylamino)naphthalene (DMAN), unlike N,N-dimethylaniline, undergoes ring metallation in n-BuLi–TMEDA–Et2O system with low selectivity and in poor total yield. The situation is significantly improved in t-BuLi–TMEDA–n-hexane system when 3- and 4-lithium derivatives become the only reaction products in good yield. The formation of 3-Li-DMAN is especially fortunate since no method of direct meta-functionalization of DMAN has been known to date. The relative stability and structure of DMAN lithium derivatives have been examined with the help of X-ray and multinuclear NMR measurements as well as DFT calculations.