M.M. Efremova, A.P. Molchanov, A.V. Stepakov, R.R. Kostikov, V.S. Shcherbakova, A.V. Ivanov
“A highly efficient [3+2] cycloaddition of nitrile oxides and azomethine imines to N-vinylpyrroles”
Tetrahedron, 2015, 71, 2071-2078
DOI:10.1016/j.tet.2015.02.058
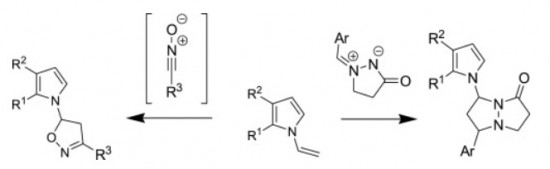
1,3-Dipolar cycloaddition of various nitrile oxides to substituted N-vinylpyrroles proceed with high efficiency and selectivity with the formation of single isomer of 5-pyrrolyl-substituted isoxazoline. The reaction of N-vinylpyrroles with cyclic azomethine imines occurs regioselectively affording 7-(pyrrol-1-yl)- substituted pyrazolo[1,2-a]pyrazolones as a mixture of two diastereomers.