M.S. Ledovskaya, A.P. Molchanov, V.M. Boitsov, R.R. Kostikov, A.V. Stepakov
“An efficient synthesis of substituted isoxazolopyrroloisoquinolines via diastereoselective N-acyliminium ion cyclization”
Tetrahedron, 2015, 71, 1952-1958
DOI:10.1016/j.tet.2015.02.031
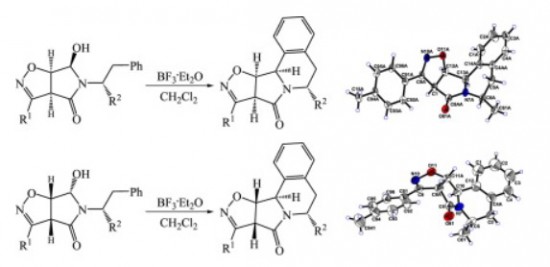
A simple and efficient strategy was developed for the synthesis of fused pyrrolo[2,1-a]isoquinoline ring systems. The 5- and 6-substituted isoxazolopyrroloisoquinolines were readily prepared via diastereoselective N-acyliminium ion cyclization of 5-(1-R(or 2-R)-substituted-2-phenylethyl)-6-hydroxytetrahydro-4H-pyrrolo[3,4-d]isoxazol-4-ones derived from the corresponding bicyclic dihydroisoxazoles.