Andreii S. Kritchenkov, Konstantin V. Luzyanin, Nadezhda A. Bokach, Maxim L. Kuznetsov, Vladislav V. Gurzhiy, Vadim Yu. Kukushkin
«Selective Nucleophilic Oxygenation of Palladium-Bound Isocyanide Ligands: Route to Imine Complexes That Serve as Efficient Catalysts for Copper-/Phosphine-Free Sonogashira Reactions»
Organometallics 2013, accepted.
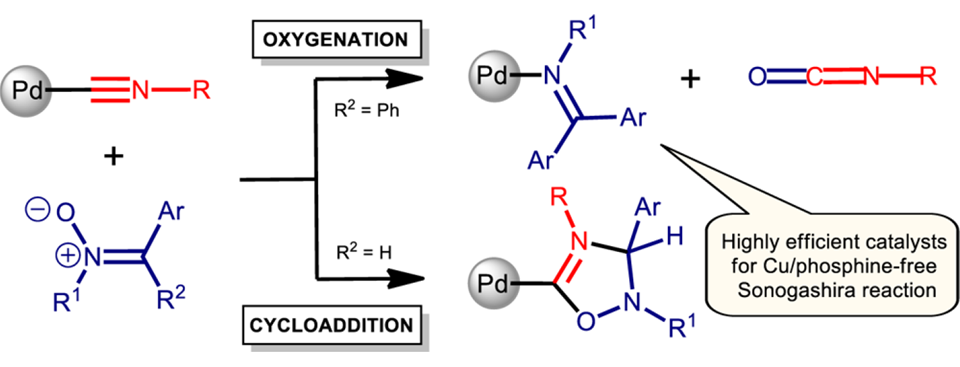
Metal-mediated reactions between cis-[PdCl2(CNR)2] (R = Xy (1), Cy (2), tBu (3), C6H3(Cl-2), Me-6 (4)) and the ketonitrones Ph2C=N(O)C6H4R′ (R′ = Me (5), Cl (6)) proceed in a 1:1 molar ratio as selective nucleophilic oxygenations and provide the imine−isocyanide complexes [PdCl2{N(C6H4R′)=CPh2}(CNR)] (7−14: R′ = Me, R = Xy (7), Cy (8), tBu (9), C6H3(Cl-2)Me-6 (10); R′ = Cl, R = Xy (11), Cy (12), tBu (13), C6H3(Cl-2)Me-6 (14)) in excellent yields (90−94%), while the reaction of the cis-[PdCl2(CNR)2] complexes with aldonitrones proceeds as 1,3-dipolar cycloaddition, giving carbene adducts which then convert to the imine complexes. Theoretical calculations at the DFT level indicate that, in the case of aldonitrones, formation of the imine complexes occurs preferably via a cycloaddition/splitting pathway, including the generation of a cycloadduct, while, in the case of ketonitrones, both the cycloaddition/splitting route and the direct oxygen atom transfer pathway are equally plausible from a kinetic viewpoint. Complexes 7−14 were characterized by elemental analyses (C, H, N), by high-resolution ESI+-MS, IR, and 1H and 13C{1H} NMR spectroscopy, and also by X-ray diffraction (for 8). The catalytic activity study conducted for 7−14, taken as the catalysts in the Cu-/phosphine-free Sonogashira reaction, was evaluated for a typical model system comprising 4-methoxyiodobenzene and phenylacetylene and affording 1-methoxy-4-(phenylethynyl)benzene. The obtained data indicate that 7−14 exhibit a high catalytic activity (yields up to 95%, TONs up to 9500), and these catalysts are among the best studied so far.