J.J. Medvedev, D.V. Semenok, X.V. Azarova, L.L. Rodina, V.A. Nikolaev
“A New Powerful Approach to Multi-Substituted 3(2H)-Furanones via Brønsted Acid-Catalyzed Reactions of 4-Diazodihydrofuran-3-ones”
Synthesis, 2016, 48, A-H
DOI:10.1055/s-0036-1588304
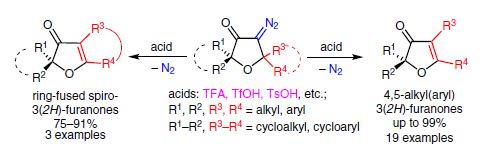
The interaction of 5,5-dialkyl(diaryl)-substituted 4-diazo-3(2H)furanones with Brønsted acids (TFA, TsOH, etc.) causes elimination of nitrogen accompanied by 1,2-nucleophilic rearrangement, giving rise to exclusive formation of 4,5-dialkyl(diaryl)-substituted 3(2Н)-furanones, ring-fused 3(2H)-furanones, and phenanthro[9,10-b]furan-3(2H)-ones in yields of up to 99%. The reaction is a new highly efficient way for the synthesis of multisubstituted 3(2Н)furanones.