E.E. Galenko, O.A. Tomashenko, A.F. Khlebnikov, M.S. Novikov
“Metal/organo relay catalysis in a one-pot synthesis of methyl 4-aminopyrrole-2-carboxylates from 5-methoxyisoxazoles and pyridinium ylides”
Org. Biomolec. Chem., 2015, 13, 9825-9833
DOI:10.1039/c5ob01537e
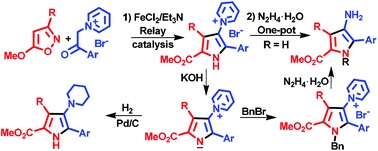
Methyl 4-aminopyrrole-2-carboxylates were synthesized in one-pot mode by the relay catalytic cascade reaction of 5-methoxyisoxazoles with pyridinium ylides by the use of a FeCl2/Et3N binary catalytic system leading to 1-(5-methoxycarbonyl-1H-pyrrol-3-yl)pyridinium salts followed by hydrazinolysis. The approach permits the introduction of a substituent at the pyrrole nitrogen via a nucleophilic reaction of the pyrrolylpyridinium ylide derived from the salt. Catalytic reduction of the ylides gives methyl 4-piperidinopyrrole-2-carboxylates.