O.A. Tomashenko, A.F. Khlebnikov, I.P Mosiagin, M.S. Novikov, E.V. Grachova, J.R. Shakirova, S.P. Tunik
“A new heterocyclic skeleton with highly tunable absorbtion/emission wavelength via H-bonding”
RSC Adv., 2015, accepted
DOI:10.1039/C5RA17755C
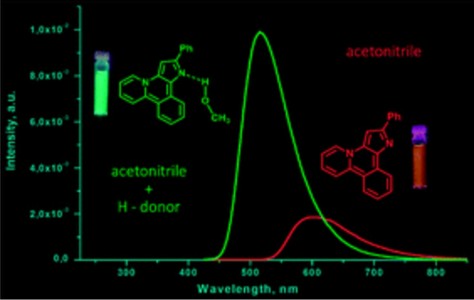
A new heterocyclic system, pyrido[2,1-a]pyrrolo[3,2-c]isoquinoline, was synthesized via Pd-catalyzed intramolecular cyclization of 1-[1-benzyl-2-(2-bromophenyl)-1H-pyrrol-3-yl]pyridin-1-ium bromides. The heterocycles obtained display stimuli responsive fluorescence in solution depending on the nature of the solvent. The strongest blue shift of the emission maxima and growth in luminescence intensity was observed in protic solvents and upon addition of proton donors to solutions of compounds in aprotic solvents. The effect of proton donors on emission characteristics was explained by DFT calculations in terms of H-complex formation with the nucleophilic centres of the molecular skeleton