A.S. Pankova, M.V. Sorokina, М.А. Кuznetsov.
“Thermal rearrangement of 2,3-diaryl-1-phthalimidoaziridines”
Tetrahedron Lett., 2015, 56,5381-5385
DOI:10.1016/j.tetlet.2015.07.093
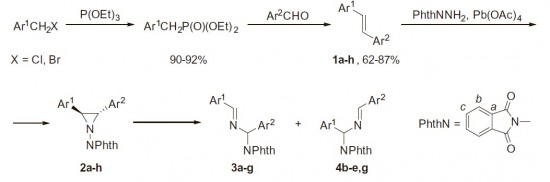
2,3-Diaryl-1-phthalimidoaziridines and 2,3-diaryl-1-phthalimidoaziridine-2-carbonitriles were found to readily undergo thermal rearrangement into imines via 1,2-migration of the phthalimido group and accompanying C–C bond cleavage. Isomerization proceeds regioselectively with preferable migration to the electron-deficient carbon atom. Interestingly, this reaction was found to predominate even in the presence of dipolarophiles.