P.R. Golubev, A.S. Pankova, M.A. Kuznetsov
“Regioselective Transition-Metal-Free Synthesis of 2‑(Trimethylsilylmethylene)pyrrol-3-ones by Thermal Cyclization of Acetylenic Enamines”
J. Org. Chem., 2015, accepted
DOI:10.1021/acs.joc.5b00398
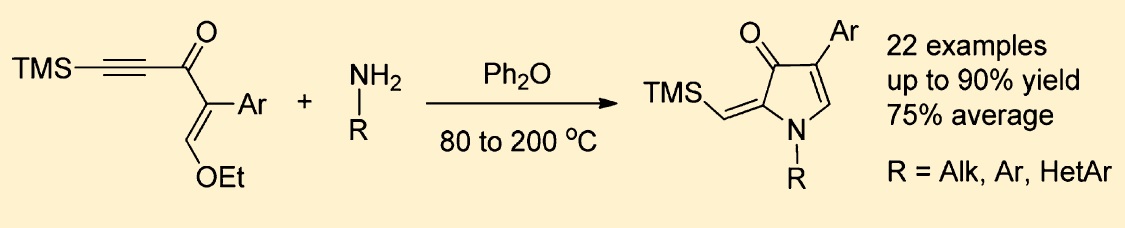
Acetylenic enamines generated in situ from readily available enynones and primary amines undergo thermal cyclization in diphenyl ether providing easy access to 4-aryl-2-(trimethylsilylmethylene)-1,2-dihydro-3H-pyrrol-3-ones. This reaction is inherently versatile, allowing for variations of substituents in both enynone and amine. Full regioselectivity along with short reaction time (1−2 h) and simple workup afford single products in good to excellent isolated yields. Fluorescent properties of the obtained compounds were studied.>