2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2013-2012
| № | Иллюстрация, выходные данные | Краткое описание основной идеи | Оборудование РЦ и результаты (по данным МР) |
|---|---|---|---|
| 1 | 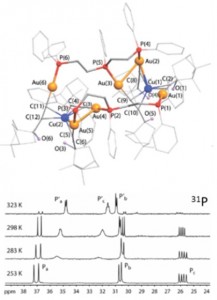 I.S. Krytchankou, D.V. Krupenya, A.J. Karttunen, S.P. Tunik, T.A. Pakkanen, P.-T. Choud, I.O. Koshevoy, “Triphosphine-supported bimetallic AuI–MI (M = Ag, Cu) alkynyl clusters” Dalton Trans., 2014, 43, 3383-3394. DOI:10.1039/C3DT52658E | The reactions of gold acetylides (AuC2R)n with triphosphine ligands PPh2-(CH2)n-PPh-(CH2)2-PPh2 (n = 1,dpmp; 2, dpep) in the presence of M+ ions (M = Cu, Ag) lead to an assembly of the heterometallic clusters, the composition of which is determined by the steric bulkiness of the alkynyl groups and the flexibility of the phosphine motifs. All the title complexes exhibit room temperature luminescence in the solid state, showing a dependence of emission energy on the structure and composition of the metal core. | Bruker Avance 400, Bruker DPX 300 spectrometers. The NMR spectroscopic investigations showed that some complexes are stereochemically non-rigid in solution and reversibly undergo possible dissociation or isomerization processes. The chemical structures of the studied compounds and complexes were confirmed by the solution 1D (1H, 31P, 13C) and 2D (COSY, HMQC, HSQC, HMBC) NMR. |
| 2 | 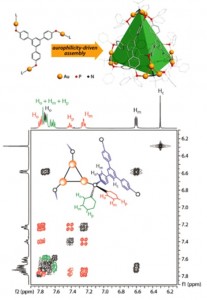 J.R. Shakirova, E.V. Grachova, A.J. Karttunen, V.V. Gurzhiy, S.P. Tunik, I.O. Koshevoy, “ Metallophilicity-assisted assembly of phosphinebased cage molecules” Dalton Trans., 2014, 43, 6236-6243. DOI:10.1039/c3dt53645a | A family of supramolecular cage molecules has been obtained via self-assembly of the phosphine–gold coordination complexes following an aurophilicity-driven aggregation approach. Use of the di- (PP) or tridentate (PPP) phosphine ligands Pn (n = 2, 3) with rigid polyaromatic backbones leads to clean formation of the coordination Pn(Au(tht))nn+ species, sequential treatment of which with H2O/NEt3 and excess of H2NBut gives the finite 3D structures of two major types: the cylindrical-like hexametallic cages and tetrahedral dodecagold complexes. | Bruker Avance 400 spectrometer. The NMR spectroscopic investigations showed that cylindrical complexes undergo twisting-like interconversion of the helical P↔M isomers in solution, while some other complexes are stereochemically rigid and retain their axially chiral architecture. The chemical structures of the studied compounds and complexes were confirmed by the solution 1D (1H, 31P) and 2D COSY NMR. |
| 3 | A.S. Pankova, M.A. Kuznetsov, “Synthesis and thermal transformations of spiro-fused N-phthalimidoaziridines” Tetrahedron Lett., 2014, 55, 2499-2503. DOI:10.1016/j.tetlet.2014.03.014 | Oxidation of N-aminophthalimide in the presence of 2-arylideneinden-1,3-diones with electron-withdrawing substituents gives the corresponding 3-aryl-1-phthalimidospiro[aziridine-2,20-indene]-10,30-diones in good yields. Heating these aziridines with standard dipolarophiles (N-phenylmaleimide, dimethyl acetylenedicarboxylate, maleate, and fumarate) leads, in most cases, to spiro[inden-2,20-pyrrole] derivatives as products of 1,3-dipolar cycloaddition of the intermediate azomethine ylides with up to 70–95% yields in the case of N-phenylmaleimide. | Bruker DPX-300, Bruker Avance 400 spectrometers. Optimal reaction conditions were found and the chemical structures (including special configuration) of the studied compounds were confirmed by the solution 1H and 2D 1H NOESY NMR. |
| 4 | 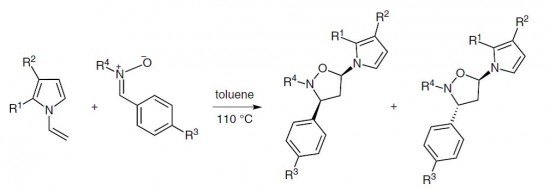 A.P. Molchanov, R.S. Savinkov, A.V. Stepakov, G.L. Starova, R.R. Kostikov, V.S. Barnakova, A.V. Ivanov, “A Highly Efficient and Stereoselective Cycloaddition of Nitrones to N-Vinylpyrroles” A.P. Molchanov, R.S. Savinkov, A.V. Stepakov, G.L. Starova, R.R. Kostikov, V.S. Barnakova, A.V. Ivanov, “A Highly Efficient and Stereoselective Cycloaddition of Nitrones to N-Vinylpyrroles”Synthesis 2014, 46, 771-780. DOI:10.1055/s-0033-1340479 | 1,3-Dipolar cycloadditions of a number of C-aryl, C-carbamoyl-, and C,C-bis(methoxycarbonyl)nitrones and substituted N-vinylpyrroles proceed with high efficiency and regioselectivity with the formation of only one isomeric substituted 5-(1H-pyrrol-1-yl)isoxazolidine cycloadduct. | Bruker DPX-300, Bruker Avance 400 spectrometers. The chemical structures of the reaction products were confirmed with the help of NMR spectroscopy. The ratio of cis/trans isomers in products was measured using 1H NMR. |
| 5 | 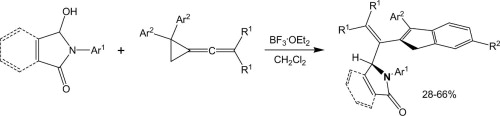 A.V. Stepakov, A.G. Larina, V.M. Boitsov, V.V. Gurzhiy, A.P. Molchanov, R.R. Kostikov, “Synthesis of indene derivatives via reactions of vinylidenecyclopropanes with the N-acyliminium cations generated from hydroxylactams” Tetrahedron Lett., 2014, 55, 2022-2026. DOI:10.1016/j.tetlet.2014.02.039 | A novel route for the synthesis of 1H-indene derivatives via the reactions of vinylidenecyclopropanes (VCPs) with the N-acyliminium cations generated from hydroxylactams is described. | Bruker DPX-300, Bruker Avance 400 spectrometers. The existence of some of the compounds as a mixture of rotamers was established by temperature-dependent NMR spectra (coalescence of exchanging signals upon heating). |
| 6 | 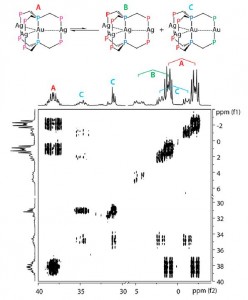 M.T. Dau, J.R. Shakirova, A.J. Karttunen, E.V. Grachova, S.P. Tunik, A.S. Melnikov, T.A. Pakkanen, I.O. Koshevoy, “Coinage Metal Complexes Supported by the Tri- and Tetraphosphine Ligands” Inorg. Chem., 2014, 53, 4705-4715. DOI:10.1021/ic500402m | A series of tri- and tetranuclear phosphine complexes of d10 metal ions supported by the polydentate ligands, bis(diphenylphosphinomethyl) phenylphosphine (PPP) and tris(diphenylphosphinomethyl) phosphine (PPPP), were synthesized. The trinuclear clusters contain a linear metal core, while in the isostructural tetranuclear complexes the metal framework has a plane star-shaped arrangement. One cluster adopts a structural motif that involves a digold unit bridged by two arms of the PPPP phosphines and decorated two spatially separated CuI ions chelated by the remaining P donors. | Bruker Avance 400 spectrometer. The 1H, 31P and COSY NMR spectroscopic investigation in DMSO solution revealed that some heterometallic clusters are stereochemically nonrigid and undergo reversible metal ions redistribution between several species, accompanied by their solvation−desolvation. |
| 7 | 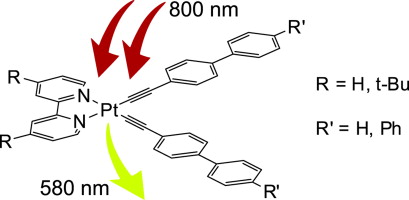 A.A. Melekhova, D.V. Krupenya, V.V. Gurzhiy, A.S. Melnikov, P.Yu. Serdobintsev, S.I. Selivanov, S.P. Tunik, “Synthesis, characterization, luminescence and non-linear optical properties of diimine platinum(II) complexes with arylacetylene ligands” J. Organomet. Chem., 2014, 763-764, 1-5. DOI:10.1016/j.jorganchem.2014.04.002 | A series of platinum(II) diimine-polyphenylacetylide complexes, which display appreciable nonlinear optical properties (TPA 9-22 GM) were synthesized and characterized. All complexes under study exhibit two-photon luminescence and their double quantum absorption cross-section was found to be in the 9-22 GM range | Bruker DPX-300 spectrometer. The structure, stoichiometry and symmetry of the complexes were confirmed by 1H and 1H COSY NMR. 1H NMR spectroscopic data indicate that in solution the complexes retain the structures found for some of them in solid state. |
| 8 | 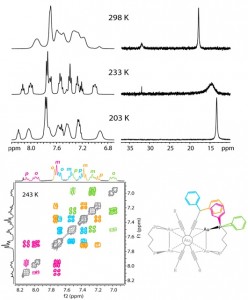 I.O. Koshevoy, Y.-C. Chang, Y.-A. Chen, A.J. Karttunen, E.V. Grachova, S.P. Tunik, J.Janis, T.A. Pakkanen, P.-T. Chou, “Luminescent Gold(I) Alkynyl Clusters Stabilized by Flexible Diphosphine Ligands” Organometallics, 2014, 33, 2363-2371. DOI:10.1021/om5002952 | Treatment of the homoleptic decanuclear compounds (AuC2R)10 with the cationic gold diphosphine complexes [Au2(PR′2-X-PR′2)2]2+ results in high-yield formation of the new family of hexanuclear clusters. All of these complexes are intensely emissive in the solid state at room temperature and demonstrate very high quantum yields from 0.61 to 1.0 with weak influence of the alkynyl substituents R′ and the diphosphine backbones on luminescence energies. | Bruker Avance 400 spectrometer. Studied compounds were characterized by 1H, 31P and 1H COSY NMR. Variable-temperature NMR spectroscopic investigations showed that all but two compounds are fluxional in solution and demonstrate dissociative chemical equilibria between major and a few minor forms. |
| 9 | 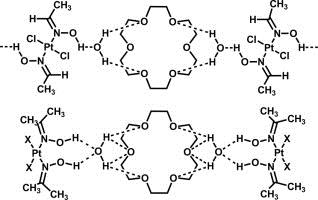 E.Yu. Bulatov, T.G. Chulkova, I.A. Boyarskaya, V.V. Kondratiev, M. Haukka, V.Yu. Kukushkin, “Triple associates based on (oxime)Pt(II) species, 18-crown-6, and water: Synthesis, structural characterization, and DFT study” J. Molec. Struct. 2014, 1068, 176-181. DOI:10.1016/j.molstruc.2014.04.010 | The associates 2(cis-[PtCl2(acetoxime)2])⋅18-crown-6⋅2H2O, 2(cis-[PtBr2(acetoxime)2])⋅18-crown-6⋅2H2O, and trans-[PtCl2(acetaldoxime)2]⋅(18-crown-6)⋅2H2O were synthesized by co-crystallization of free corresponding platinum species. The (oxime)Pt(II) species are assembled with 18-crown-6 and water by hydrogen bonding between the hydroxylic hydrogen atoms of the oxime ligands and the oxygen atom of water and between the hydrogen atoms of water and the oxygen atoms of 18-crown-6. and were characterized by 1H NMR and by and IR spectroscopies, high-resolution mass-spectrometry (ESI), TG/DTA, and X-ray crystallography. | Bruker DPX-300 spectrometer. Studied associates were characterized by 1H NMR spectroscopy. |
| 10 | L.L. Rodina, J.J. Medvedev, O.S. Galkina, V.A. Nikolaev, “Thermolysis of 4-Diazotetrahydrofuran-3-ones: Total Change of Reaction Course Compared to Photolysis” Eur. J. Org. Chem. 2014, 14, 2993-3000. DOI:10.1002/ejoc.201400161 | Thermolysis of 2,2,5,5-tetrasubstituted 4-diazodihydrofuran-3-ones in protic (BnOH) and aprotic (DMSO) media, in contrast to photolysis, gives rise to the formation of 2,2,4,5-substituted 3(2H)-furanones as a result of 1,2-alkyl (aryl) shift. This is a first-order reaction that furnishes higher yields of furanones than thermolysis in BnOH. The reaction can serve as a preparative method for the synthesis of tetrasubstituted-3(2H)-furanones. | Bruker DPX-300, Bruker Avance 400 spectrometers. The chemical structures of the studied compounds in DMSO solution, kinetics of thermolysis and yields of products were determined by 1H and 13C NMR. |
| 11 | M.A. Kinzhalov, K.V. Luzyanin, I.A. Boyarskaya, G.L. Starova, V.P. Boyarskiy, “Synthetic and structural investigation of [PdBr2(CNR)2] (R = Cy, Xyl)” J Molec. Struct. 2014, 1068, 222-227 DOI: 10.1016/j.molstruc.2014.04.025 | Сomplexes trans-[PdBr2(CNR)2] (R = Cy, Xyl) were prepared by the metathetical reaction between the corresponding cis-dichloro derivatives with excess KBr in acetone and fully characterized by several spectroscopic techniques. Experimental and theoretical data are in agreement that trans coordination modes are preferable for dibromo species as in gas phase or in solution and in a solid state. In contrast, the dichloro complex [PdCl2(CNCy)2], was found to be more stable as trans-form in gas phase and cis-form in solution as well as in solid state. | Bruker Avance 400 NMR spectrometer. The compounds were characterized by 1H and 13C{1H} and DEPT NMR spectroscopy. |
| 12 | 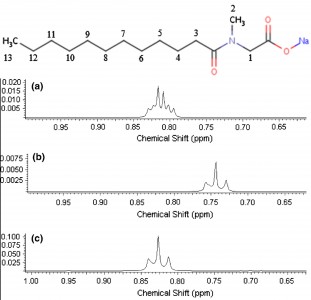 M.V. Popova, D. Michel, “Behavior of Sodium Lauroyl Sarcosinate in Solution and Binary Mixtures by Means NMR” Appl. Magn. Reson. 2014, 45, 353-364 DOI: 10.1007/s00723-014-0531-9 | Measurements of the 1H and 13C chemical shifts as well as 1H spin–lattice relaxation times of sodium lauroyl sarcosinate (SLAS) in aqueous solutions and mixed binary systems with co-surfactants were carried out at various concentrations. It is shown that changes in the chemical shifts for the N–CH2 groups in SLAS with increasing surfactant concentration can be used to estimate the ratio pcis/ptrans of cis- and trans-isomers. | 1H and 13C NMR experiments were performed on a Bruker Avance 500 NMR spectrometer. The structure and dynamics of SLAS were investigated in binary solution and mixed surfactant systems at different concentrations by means 1H NMR spectroscopy and relaxation. |
| 13 | 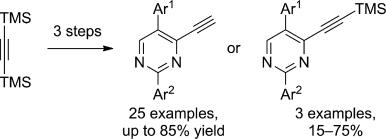 P.R. Golubev, A.S. Pankova, M.A. Kuznetsov, “Transition-Metal-Free Approach to 4-Ethynylpyrimidines via Alkenynones” Eur. J. Org. Chem. 2014, 3614-3621 DOI: 10.1002/ejoc.201402045 | A practical approach to the synthesis of 4-ethynylpyrimidines by the condensation of arylamidines with 2-aryl-1-ethoxy-5-(trimethylsilyl)pent-1-en-4-yn-3-ones has been developed. As these latter ketones are easily accessible from bis(tri-methylsilyl)acetylene and arylacetyl chlorides, the regioselective condensation reported herein provides a facile access to both TMS-protected and unprotected 4-ethynylpyrimidines in yields of up to 85%. | NMR spectroscopic data were recorded with Bruker DPX-300 and Avance 400 spectrometers. NMR spectroscopic data of all the investigated pyrimidines revealed that they show a very consistent set of signals from the core molecule. |
| 14 |  J. Malinina, T.Q. Tran, A.V. Stepakov, V.V. Gurzhiy, G.L. Starova, R.R. Kostikov, A.P. Molchanov, “[3+2] Cycloaddition reactions of arylallenes with C-(N-arylcarbamoyl)- and C,C-bis(methoxycarbonyl)nitrones and subsequent rearrangements” Tetrahedron Lett. 2014, 55, 3663-3666 DOI: 10.1016/j.tetlet.2014.04.107 | The first example of 1,3-dipolar cycloaddition reactions of nitrones with arylallenes is described. C-Carbamoylnitrones react with the C1-C2 double bond of arylallenes affording the corresponding 4-methyleneisoxazolidines in good yields. | 1H and 13C NMR spectra were recorded in CDCl3 as a solvent using a Bruker DPX–300 or Bruker Avance 400 spectrometer for the confirmation of the molecular structure. |
| 15 | 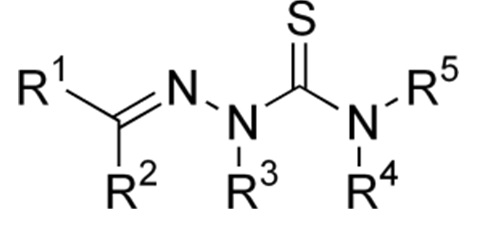 M.A. Kuznetsov, A.Ya. Bespalov,”One-pot, Three-component Synthesis of [1,3]thiazolo[4,3-b][1,3,4]thiadiazoles: Correct Structure of the Products” Chem. Hetercycl. Compd. 2014, 49, 1458-1463. DOI: 10.1007/s10593-014-1396-4 | The products of the one-pot, three-component synthesis of [1,3]thiazolo-[4,3-b][1, 3, 4]thiadiazoles from aromatic aldehydes, thioglycolic acid, and compounds containing a C(=S)–N–NH2 fragment (thiosemicarbazide or 4-amino-2,4-dihydro-3H-1,2,4-triazole-3-thiones) are not condensed hetero-cycles (as reported by several researchers), but are thiosemicarbazones or triazolylimines of the aldehydes used. | Bruker Avance 400 spectrometer. 1H and 13C NMR spectra clearly contradict the condensed heterocyclic structure, proposed earlier in the literature, but are consistent with the thiosemicarbazones or triazolylimines of the aldehydes used. |
| 16 | 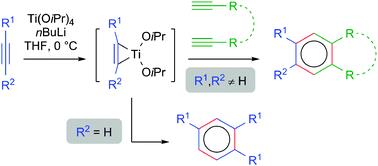 V.A. Rassadin, E. Nicolas, Y. Six, “Ti(OiPr)4/nBuLi: an attractive reagent system for [2+2+2] cyclotrimerisation reactions” Chem. Commun. 2014, 50, 7666-7779. DOI: 10.1039/C4CC02698E | A convenient method for the [2+2+2] cyclotrimerisation of alkynes using Ti(OiPr)4/nBuLi is presented. Homotrimerisation of arylacetylenes proceeds within minutes with excellent regioselectivity. Moreover, the intermolecular construction of ABB heterotrimers can be achieved selectively fromtwo different alkynes with similar electronic properties. The method is also suitable for the synthesis of pyridines. | Bruker Avance 400 spectrometer. The structures of the studied compounds and especially the presence and the fractions of their regioisomers was confirmed by NMR. |
| 17 |  V.V. Sokolov, A.Yu. Ivanov, M.S. Avdontseva, A.A. Zolotarev, “Stereochemistry and NMR Spectra of Some Tricyclic Condensed Thiazolidine Derivatives with a Bridgehead Nitrogen Atom” Chem. Hetercycl. Compd. 2014, 50, 550-556. DOI: 10.1007/s10593-014-1506-3 | The configuration of a series of tricyclic condensed thiazolidines with a bridgehead nitrogen atom, for which erroneous data had been published, was determined by X-ray structural analysis and NMR spectroscopy. | Bruker DPX-300, Bruker Avance 400, Bruker Avance 500 spectrometers. The spacial structures of studied compounds were established reliably using an array of liquid-state NMR methods: 1H ,13C, 1D TOCSY, 1D NOESY, 2D HSQC etc. |
| 18 |  P.B. Davidovich, D.S. Novikova, V.G. Tribulovich, S.N. Smirnov, V.V. Gurzhiy, G. Melino, A.V. Garabadzhiu,”First X-ray Structural Characterization of Isatin Schiff-Base Derivative. NMR and Theoretical Conformational Studies” J. Molec. Struct. 2014, 1075, 450-455. DOI: 10.1016/j.molstruc.2014.07.008 | Isatin (1H-indole-2,3-dione) is an endogenous natural compound under intense development in medicinal chemistry. In this paper isatin Schiff base derivative is characterized by X-ray, NMR, FT-IR, UV-Vis spectroscopy and by quantum chemistry calculations. | Bruker Avance 400, Bruker Avance 500 spectrometers. All NMR assignments and stereochemistry of the studied compounds were made on the basis of 1D (1H, 13C) and 2D NMR experiments (geCOSY, geNOESY, geHSQC, geHMBC). NMR data show that E-conformer interconverts to the Z-conformer when dissolved, this equilibrium weakly depends on the solvent type. |
| 19 |  A. Miroslavov, Y. Polotskii, V. Gurzhiy, A. Ivanov, A. Lumpov, M. Tyupina, G. Sidorenko, P. Tolstoy, D. Maltsev, D. Suglobov, «Technetium and Rhenium Pentacarbonyl Complexes with C2 and C11 ω-Isocyanocarboxylic Acid Esters» Inorg. Chem. 2014, 53(15), 7861-7869. DOI: 10.1021/ic500327s | Technetium(I) and rhenium(I) pentacarbonyl complexes with ethyl 2-isocyanoacetate and methyl 11-isocyanoundecanoate, were prepared and characterized by IR, 1H NMR, and 13C{1H} NMR spectroscopy. | The 1H and 13C{1H} NMR spectra were recorded on a Bruker Avance III 400. NMR characterized technetium(I) and rhenium(I) pentacarbonyl complexes. |
| 20 |  Dz.N. Konshina, A.V. Furina, Z.A. Temerdashev, A.A. Gurinov, V.V. Konshin, “Immobilization of Guanazyl Functional Groups on Silica for Solid-Phase Extraction of Metal Ions” Analytical Lett. 2014, 47(16), 2665-2681. DOI: 10.1080/00032719.2014.917421 | Guanazyl groups were grafted on silica gel by 4-nitrobenzoyl chloride acylation, sodium dithionite reduction, diazotation, and reaction with 2-benzylidenehydrazinecarboximidamide. The applicability of pseudosecond order kinetic equations for metal sorption kinetics description was investigated. The method was employed for the determination of Cu(II) in water with a low limit of detection, high accuracy, and good precision. | 13С{1H}CP MAS NMR spectra were measured using a 400 MHz Bruker WB Avance III spectrometer. The two dimensional heteronuclear correlation experiment confirmed the presence of the carboxamide and guanyl carbons and showed cross-peaks between carbon and hydrogen atoms in the methylene and phenyl groups. |
| 21 |  A.V. Stepakov, V.M. Boitsov, A.G. Larina, A.P. Molchanov, ”Acid-induced rearrangement of cycloadducts from N-aryl itaconimides and 1,3-diphenylisobenzofuran” Tetrahedron Lett. 2014, 55, 4895-4897 DOI: 10.1016/j.tetlet.2014.06.107 | Treatment of several Diels–Alder adducts of N-aryl itaconimides and 1,3-diphenylisobenzofuran with a strong acid triggers a skeletal rearrangement resulting in 2-aryl-6,10b-diphenylbenzo[h]isoquinoline-1,3(2H,10bH)-diones. | The 1H and 13C NMR spectra were measured on a Bruker DPX-300 and a Bruker Avance II 400. |
| 22 |  D.S. Ryabukhin, L.Yu. Gurskaya, G.K. Fukin, A.V. Vasilyev, “Superelectrophilic activation of N-aryl amides of 3-arylpropynoic acids: synthesis of quinolin-2(1H)-one derivatives” Tetrahedron 2014, 70, 6428-6443 DOI: 10.1016/j.tet.2014.07.028 | Reported that the superelectrophilic activation of N-aryl amides of 3-arylpropynoic acids by Bronsted superacids (CF3SO3H, HSO3F) or strong Lewis acids AlX3 (X¼Cl, Br) results in the formation of 4-aryl quinolin-2(1H)-ones in quantitative yields. | The 1H, 13C, 19F NMR spectra of compounds solutions in CDCl3 were recorded on a Bruker-400 spectrometer. Structures of reactants, intermediate and final products of chemical reactions were confirmed. |
| 23 |  O.Yu. Bakulina, A.Yu. Ivanov, D.V. Dar’in, P.S. Lobanov, “New transformations of 2-methylsulfanyl-4,6-dichloropyrimidine-5-carbaldehyde involving enamines: synthesis of condensed azines” Mendeleev Commun. 2014, 24, 163-164. DOI: 10.1016/j.mencom.2014.04.013 | The reaction between 4,6-dichloropyrimidine-5-carbaldehyde and methyl 3-aminocrotonate leads to pyrido[2,3-d]pyrimidine which reacts with ethyl 3,3-diaminoacrylate to give pyrimido[4,5,6-de][1,6]naphthyridine derivative. | 1H and 13C NMR spectra were recorded on a Bruker Avance III 400 spectrometer. The chemical structures of the studied compounds were confirmed by NMR. |
| 24 | 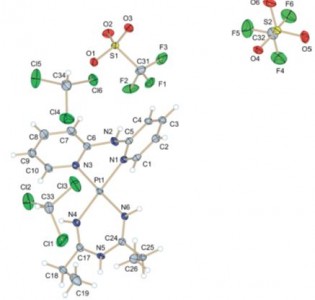 I.I. Eliseev, P.V. Gushchin, Yi.-A. Chen, P.-T. Chou, M. Haukka, G.L. Starova, V.Yu. Kukushkin, “Phosphorescent Pt(II) Systems Featuring Both 2,2’-Dipyridylamine and 1,3,5-Triazapentadiene Ligands” Eur. J. Inorg. Chem. 2014, 4101-4108 DOI: 10.1002/ejic.201402364 | The treatment of cis-[Pt(dpa)(RCN)2][SO3CF3]2 {dpa = 2,2-dipyridylamine, R = Me, Et, CH2Ph, Ph; [2a–d](OTf)2} (OTf = SO3CF3) with 2 equiv. of N,N-diphenylguanidine [NH=C-(NHPh)2] in CH2Cl2 solutions gives [Pt(dpa){NH=C(R)NC(NHPh)=NPh}][SO3CF3] {[3a,b,d](OTf)} as the addition products and [Pt(dpa){NH=C(R)NHC(R)=NH}][SO3CF3]2 {[4a,b](OTf)2} as the tailoring products. The resultant complexes are nonemissive in solution, mainly because of the interaction between the empty dz2 orbital in a square-planar configuration and solvent molecules. However, in the solid state, some complexes exhibit strong phosphorescence with quantum yields. | Bruker DPX-300 NMR spectrometer. Structure and composition of the studied molecules was supported by 1H, 13C{1H} and 1H-1H COSY NMR spectra. |
| 25 | 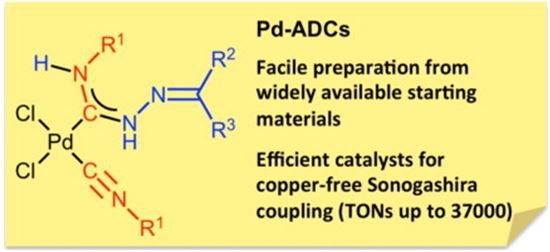 E.A. Valishina, M.F.C. Guedes da Silva, M.A. Kinzhalov, S.A. Timofeeva, T.M. Buslaeva, M. Haukka, A.J.L. Pombeiro, V.P. Boyarskiy, V.Yu. Kukushkin, K.V. Luzyanin, “Palladium-ADC complexes as efficient catalysts in copper-free and room temperature Sonogashira coupling” J. Molec. Catalysis A 2014, 395, 162-171 DOI: 10.1016/j.molcata.2014.08.018 | The metal-mediated coupling between cis-[PdCl2(CNR1)2] [R1 = cyclohexyl (Cy), t-Bu,2,6-Me2C6H3(Xyl) 3, 2-Cl-6-MeC6H3] and hydrazones H2NN CR2R3[R2,R3= Ph; R2,R3= C6H4(OMe-4); R2/R3= 9-fluorenyl; R2 = H, R3 = C6H4(OH-2)] provided carbene complexescis-[PdCl2{C(N(H)N CR2R3) N(H)R1}(CNR1)] in good yields. | Bruker Avance 400 and Bruker Avance 500 spectrometers. 1H NMR, 1D (1H,13C{1H}) and 2D (1H,1H-COSY, 1H,13C-HMQC/1H,13C-HSQC, 1H,13C-HMBC) NMR spectra were recorded to elucidate structures. |
| 26 | 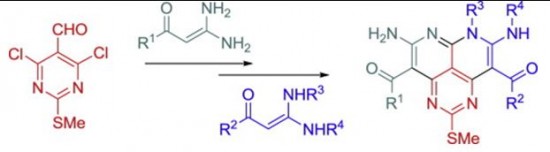 O.Yu. Bakulina, A.Yu. Ivanov, P.S. Lobanov, D.V. Dar’in, “Synthesis of novel peri-fused heterocyclic systemsdpyrimido [4,5,6-de][1,8]naphthyridines, based on interaction of 4,6-dichloro-2-methylthiopyrimidine-5-carbaldehyde with geminal enediamines” Tetrahedron 2014, 70, 7900-7905 DOI: 10.1016/j.tet.2014.08.066 | The synthetic approach to novel peri-fused heterocyclic systems – pyrimido[4,5,6-de][1,8]naphthyridines, has been developed. It consists of successive treatment of 4,6-dichloro-2-methylthiopyrimidine-5-carbaldehyde with 2 mol of geminal b-(acyl)enediamines and includes substitution of a chlorine atom with the nucleophilic carbon atom of the enediamine and cyclization of the corresponding intermediate. The possibility of synthesis of both symmetrically and nonsymmetrically substituted pyrimidonaphthyridines was demonstrated. | The NMR spectra were recorded on a Bruker Avance III 400 spectrometer. Structures were confirmed by NMR spectroscopy, including 1H, 13C, HMBC and HSQC spectra. The significant broadening of the ring carbon atoms signals in the 13C NMR spectrum demonstrates the tautomeric equilibrium in pyrimidonaphthyridines. |
| 27 | 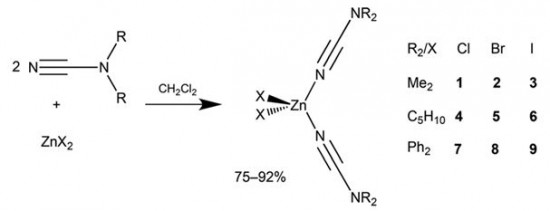 A.S. Smirnov, E.S. Butukhanova, N.A. Bokach, G.L. Starova, V.V. Gurzhiy, M.L. Kuznetsov, V.Yu. Kukushkin, “Novel (cyanamide)ZnII complexes and zinc(II)-mediated hydration of the cyanamide ligands” Dalton Trans. 2014, 43, 15798-15811 DOI: 10.1039/c4dt01812e | The cyanamides NCNR2 (R2 = Me2, Ph2, C5H10) react with ZnX2 (X = Cl, Br, I) in a 2:1 molar ratio at room temperature, giving a family of zinc(II) complexes [ZnX2(NCNR2)2] (R2 = Me2, X = Cl, X = Br , X = I; R2 = C5H10, X = Cl, X = Br; X = I; R2 = Ph2, X = Cl, X = Br, X = I; 75–92% yields). | 1H, 13C{1H}, and 19F{1H} NMR spectra were acquired on a Bruker 400 MHz Avance spectrometer at ambient temperature to elucidate the structure of complexes. |
| 28 | 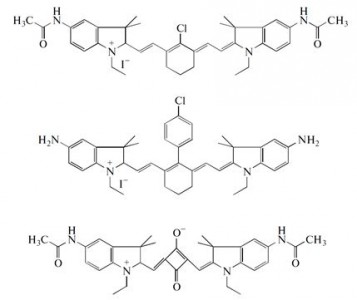 M.Ya. Goikhman, N.P. Yevlampieva, I.V. Podeshvo, S.A. Mil’tsov, V.S. Karavan, I.V. Gofman, A.P. Khurchak, A.V. Yakimansky, “Polymers with Cyanine Chromophore Groups in the Main Chain: Synthesis and Properties”; Polymer Science B, 2014, 56, 352-359 DOI: 10.1134/S1560090414030051 | Polyamides containing fragments of two cyanine chromophores in the main chain are synthesized, and their viscometric and electrooptical properties in solutions, as well as their stress–strain properties in films, are investigated. It is shown that the molecular characteristics of the copolyamides are substantially affected by chromophore fragments at a content of 10 mol %, while the mechanical properties of the films are independent of the chemical structures of chromophores incorporated into polyamide chains. | Solution-state 1H NMR spectra were recorded on a Bruker DPX 300 spectrometer to characterize the structure and conformation of cyanine chromophores. |
| 29 | 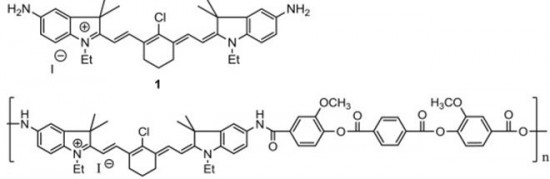 S. Miltsov, V. Karavan, M. Goikhman, I. Podeshvo, S. Gómez-de Pedro, M. Puyol, J. Alonso-Chamarro, “Synthesis of bis-aminosubstituted indocyanine dyes for their use in polymeric compositions” Dyes and Pigments, 2014, 109, 34-41 DOI: 10.1016/j.dyepig.2014.05.002 | The synthesis of a set of open-chain bis-aminosubstituted cyanine dyes as well as others with cyclic fragments in the polymethine chain is presented. These dyes are suitable for the development of polymeric compositions with variable optical characteristics as they can be covalently incorporated into the polymer. Bis-aminosubstituted cyanine dyes with cyclic fragments in the polymethine chain as well as open-chained were synthesized. All them are potential chromophores to be employed in the development of chromophore-containing polymeric systems by their covalently incorporation into the main or the side chains of the polymer. In this way, problems related to solubility, heterogeneous distribution, stability or low optical quality present in typical “guest”/“host” systems could be avoided. | 1H NMR spectra have been measured in a Bruker DPX-300 at 300 MHz to characterize bis-aminosubstituted cyanine dyes with cyclic fragments in the polymethine chain as well as open-chained. |
| 30 | 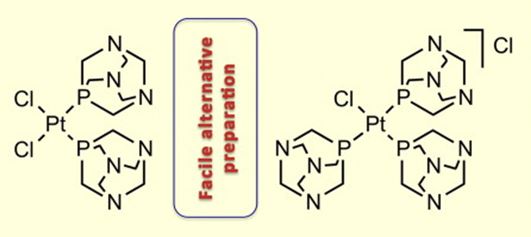 M.Ya. Demakova, K.V. Luzyanin, G.L. Starova, V.Yu. Kukushkin, “Facile alternative route to cis-[PtCl2(PTA)2] and [PtCl(PTA)3]Cl (PTA = 1,3,5-triaza-7-phosphaadamantane)” Inorg. Chem. Commun., 2014, 50, 17-18 DOI: 10.1016/j.inoche.2014.10.002 | The reaction of trans-[PtCl2(Me2SO)2] with 2 equivs of 1,3,5-triaza-7-phosphaadamantane (PTA) in MeNO2 at RT furnished cis-[PtCl2(PTA)2] in 87% isolated yield. Corresponding reaction of K2[PtCl4] with 1 equiv. of PTA in aqueous EtOH at RT led to [PtCl(PTA)3]Cl in 84% isolated yield. Complexes were characterized by elemental analyses,HR-ESI+/−-MS, IR, 1H and 31P{1H} NMR spectroscopic techniques, and by single-crystal X-ray diffraction for cis-[PtCl2(PTA)2]. | Bruker Avance 400 NMR spectrometer. 1H and 31P{1H} NMR spectra were used for characterization of complexes. |
| 31 | 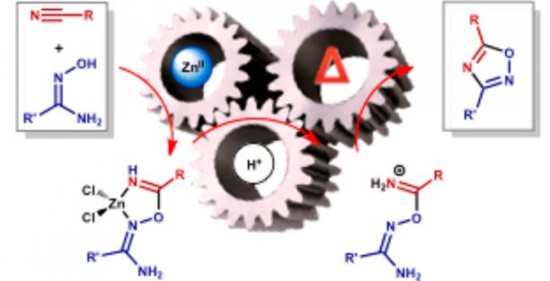 D.S. Bolotin, K.I. Kulish, N.A. Bokach, G.L. Starova, V.V. Gurzhiy, V.Yu. Kukushkin, “Zinc(II)-Mediated Nitrile−Amidoxime Coupling Gives New Insights into H+-Assisted Generation of 1,2,4-Oxadiazoles” Inorg. Chem., 2014, 53, 10312-10324 DOI: 10.1021/ic501333s | The cyanamides Me2NCN, OC4H8NCN, and PhC(=O)N(H)CN and the conventional nitriles PhCN and EtCN react with 1 equiv of each of the amidoximes R′C(=NOH)NH2 (R′ = Me), Ph )) in the presence of 1 equiv of ZnCl2 producing the complexes [ZnCl2{HN=C(R)ON=C(R′)NH2}] (R/R′ =NMe2/Me), NMe2/Ph, NC4H8O/Me, NC4H8O/Ph, N(H)C(=O)Ph/Me, N(H)C(=O)Ph/Ph, Ph/Me, Ph/Ph, Et/Ph) with the chelate ligands originating from the previously unreported zinc(II)-mediated nitrile−amidoxime coupling. | Solid-state 13C MAS spectra were measured on Bruker Avance III 400WB NMR spectrometer for the confirmation of the structure of the studied compounds. Additional reactions run as “blank” experiments monitored by 1H NMR point out that the observed nitrile−amidoxime coupling is zinc(II)-mediated. |
| 32 | 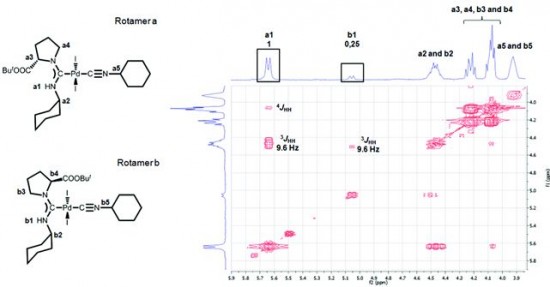 T.B. Anisimova, M. Fátima C. Guedes da Silva, V.Yu. Kukushkin, A.J. L. Pombeiro, K.V. Luzyanin, “Metal-mediated coupling of amino acid esters with isocyanides leading to new chiral acyclic aminocarbene complexes” Dalton Trans., 2014, 43, 15861-15871 DOI: 10.1039/c4dt01917b | Metal-mediated coupling between equimolar amounts of cis-[PdCl2(CNR1)2] and the amino acid esters L-HTyrOMe or L-HProOtBu led to the formation of the complexes cis-[PdCl2(CNR1){C(TyrOMe)=NHR1}] (R1 = Xyl, 2-Cl-6-Me-C6H3) or cis-[PdCl2(CNR1){C(ProOtBu)=NHR1}] (R1 = Xyl, 2-Cl-6-Me-C6H3, Cy, tBu, 2-naphthyl) in good to excellent isolated yields (75–94%). | Bruker Avance III 400 and 500 spectrometers. 1D (1H, 13C{H}) and 2D (COSY, HMQC, HSQC, HMBC) NMR techniques were used for characterization of some complexes. |
| 33 | 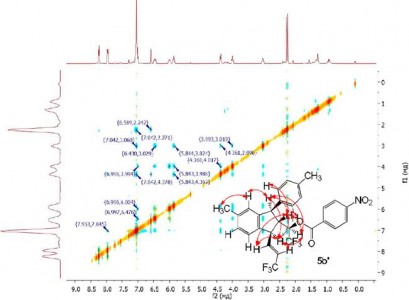 A.N. Kazakova, R.O. Iakovenko, V.M. Muzalevskiy, I.A. Boyarskaya, M.S. Avdontceva, G.L. Starova, A.V. Vasilyev, V.G. Nenajdenko, “Trifluoromethylated allyl alcohols: acid-promoted reactions with arenes and unusual ‘dimerization’”; Tetrahedron Lett., 2014, 55, 6851-6855 DOI: 10.1016/j.tetlet.2014.10.083 | An unusual ‘dimerization’ of CF3-allyl alcohols [ArCH=CHCH(OH)CF3] under the action of anhydrous FeCl3 was found to give fluorinated indanes in 62–90% yields via the formation of intermediate allyl cations. Reactions of CF3-allyl alcohols with arenes (Ar′H) led to CF3-alkenes [Ar(Ar′)CHCH=CHCF3] in 48–75% yields. The mechanisms of the transformations are discussed. | Bruker Avance III 400 spectrometer. Structure and stereochemistry of studied compounds were characterized 1Н NMR, 19F NMR, 13C NMR, H-H NOESY, H-F NOESY and DEPT spectra. |
| 34 | 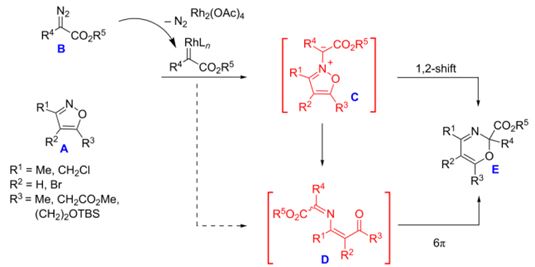 A.F. Khlebnikov, M.S. Novikov, Y.G. Gorbunova, E.E. Galenko, K.I. Mikhailov, V.V. Pakalnis, M.S. Avdontceva, “Isoxazolium N-ylides and 1-oxa-5-azahexa-1,3,5-trienes on the way from isoxazoles to 2H-1,3-oxazines” Beilstein J. Org. Chem. 2014, 10, 1896-1905 DOI: 10.3762/bjoc.10.197 | Theoretical and experimental studies of the reaction of isoxazoles with diazo compounds show that the formation of 2H-1,3-oxazines proceeds via the formation of (3Z)-1-oxa-5-azahexa-1,3,5-trienes which undergo a 6π-cyclization. Reaction conditions were found which allow for the preparation of aryl- and halogen-substituted 2H-1,3-oxazines as well as 1,4-di(alkoxycarbonyl)-2-azabuta-1,3-dienes starting from isoxazoles and diazo esters. | NMR spectra were recorded with a Bruker DPX 300 and a Bruker Avance III 400 spectrometer. 1H and 13C NMR spectra in CDCl3 solution were used to verify the structures of some compounds. |
| 35 | 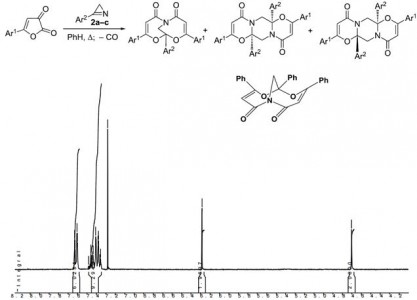 A.F. Khlebnikov, M.S. Novikov, V.V. Pakalnis, R.O. Iakovenko, D.S. Yufit, “Domino reactions of 2H-azirines with acylketenes from furan-2,3-diones: Competition between the formation of ortho-fused and bridged heterocyclic systems” Beilstein J. Org. Chem. 2014, 10, 784–793. DOI: 10.3762/bjoc.10.74 | 3-Aryl-2H-azirines react with acylketenes, generated by thermolysis of 5-arylfuran-2,3-diones, to give bridged 5,7-dioxa-1-azabicyclo[4.4.1]undeca-3,8-diene-2,10-diones and/or ortho-fused 6,6a,12,12a-tetrahydrobis[1,3]oxazino[3,2-a:3′,2′-d]pyrazine-4,10-diones. The latter compounds, with a new heterocyclic skeleton, are the result of the coupling of two molecules of azirine and two molecules of acylketene and can be prepared only from 3-aryl-2H-azirines having no electron-withdrawing groups in the aryl substituent. | The 1H and 13C NMR spectra were measured on a Bruker Avance II 400. Characterization data for all synthesized compounds were obtained by 1H and 13C NMR spectra for all new compounds. |
| 36 | 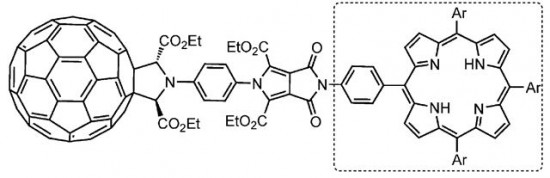 A.S. Konev, A.F. Khlebnikov, P.I. Prolubnikov, A.S. Mereshchenko, A.V. Povolotskiy, O.V. Levin, A. Hirsch, “Synthesis of New Porphyrin–Fullerene Dyads Capable of Forming Charge-Separated States on a Microsecond Lifetime Scale” Chem. Eur. J. 2014, 20,1-15 DOI: 10.1002/chem.201404435 | A series of covalently linked axially symmetric porphyrin–fullerene dyads with a rigid pyrrolo[3,4-c]pyrrolic linker enabling a fixed and orthogonal arrangement of the chromophores has been synthesized and studied by means of transient absorption spectroscopy and cyclic voltammetry. The lifetime of the charge-separated state has been found to depend on the substituents on the porphyrin core, reaching up to 4 μs for a species with meso-(p-MeOC6H4) substituents. | Bruker Avance 400 and Bruker DPX-300 NMR spectrometers. The structures were confirmed by 1H and 13C NMR spectra using CDCl3, [D6]DMSO, or C6D6 as solvents. |
| 37 |  N.A. Danilkina, A.E. Kulyashova, A.F. Khlebnikov, S. Bräse, I.A. Balova, “Electrophilic Cyclization of Aryldiacetylenes in the Synthesis of Functionalized Enediynes Fused to a Heterocyclic Core” J. Org. Chem. 2014, 79(19), 9018-9045 DOI: 10.1021/jo501396s | An efficient strategy for the synthesis of asymmetrically substituted enediynes fused to benzothiophene, benzofuran, and indole was developed. These substrates and their modified derivatives were easily converted by Sonogashira coupling with acetylenes to a variety of asymmetrically substituted acyclic enediynes fused to heterocycles. The tolerance of the developed methodology to a variety of functional groups is a great advantage in the synthesis of macrocyclic enediyne systems fused to a heterocyclic core. Synthesis of indole-fused 12-membered macrocyclic dienediyne was achieved using ring-closing metathesis as a key step. | Bruker Avance 400 and Bruker DPX-300 spectrometers were used. 1H and 13C NMR spectra for all synthesized compounds were recorded. The structure determination was made for some compounds by 2D NMR. |
| 38 |  A.F. Khlebnikov, O.A. Tomashenko, L.D. Funt, M.S. Novikov, “Simple Approach to Pyrrolylimidazole Derivatives by Azirine Ring Expansion with Imidazolium Ylides” Org. Biomol. Chem. 2014, 12, 6598-6609 DOI: 10.1039/c4ob00865k | A domino reaction of 2H-azirines with 1-alkyl-3-phenacyl-1H-imidazolium bromides in the presence of Et3N provides a facile route to 1-alkyl-3-(1H-pyrrol-3-yl)-1H-imidazol-3-ium bromides presented. Very simple procedures used for isolation of products do not involve chromatography. The investigated compounds exist in the ylide form in solution and in the solid state, which is in agreement with the relative stabilities of the species calculated with the PCM solvent model. | The structures were confirmed by NMR method. 1H and 13C NMR spectra were recorded on a Bruker 400 MHz Avance spectrometer. |
| 39 |  A.S. Konev, D.A. Lukyanov, P.S. Vlasov, O.V. Levin, A.A. Virtsev, I.M. Kislyakov, A.F. Khlebnikov, “The Implication of 1,3-Dipolar Cycloaddition of Azomethine Ylides to the Synthesis of Main-Chain Porphyrin Oligomers” Macromol. Chem. Phys. 2014, 215, 516-529 DOI: 10.1002/macp.201300679 | The polycycloaddition of azomethine ylides to dipolarophiles was described for the first time. Use of (cis,cis)-bis-aziridines as a source of azomethine ylides allows selective polyaddition to be realized, which leads to oligomers exclusively with trans-substituted pyrrolidine rings and modest molecular weights. | Bruker DPX-300 and Bruker Avance 400 NMR spectrometer. The consistence of the structures was examined by 1H and 13C NMR data. 2D NMR spectroscopy differentiated porphyrins in cis and trans isomers. |
| 40 | 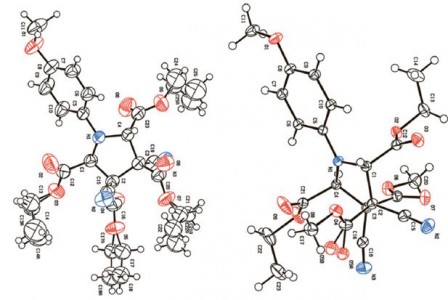 A.F. Khlebnikov, A.S. Koneva, A.A. Virtseva, D.S. Yufit, G. Mloston, H. Heimgartner, “Concerted vs. non-concerted 1,3-dipolar cycloadditions of azomethine ylides to electron-deficient dialkyl 2,3-dicyanobut-2-enedioates” Helv. Chim. Acta 2014, 97, 453-470 DOI: 10.1002/hlca.201300405 | The quantum-chemical calculations of the thermal ring opening of 1-methyl-2,3-diphenyl- and 1,2,3-triphenylaziridine with formation of the corresponding azomethine ylides of S-, U-, and W-type as well as their cycloaddition to dimethyl acetylenedicarboxylate (DMAD) and dimethyl 2,3-dicyanobut-2-enedioate, were performed at the DFT B3LYP/6-31G(d) level of theory with the PCM solvation model. The calculations are in complete accordance with experimental results and explain the switch from the concerted to the non-concerted pathway depending on substituents in the dipolarophile and the ylide. | 1H- and 13C NMR spectra were obtained on a Bruker DPX-300 spectrometer. NMR spectra were used to determine the ratio of cycloadducts in the reactions of cis- or trans isomers. |
| 41 | 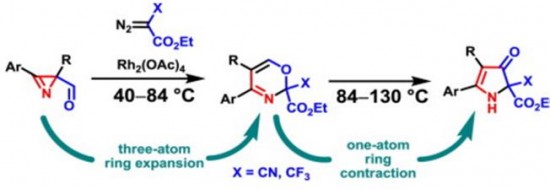 K.V. Zavyalov, M.S. Novikov, A.F. Khlebnikov, V.V. Pakalnis, “Selective syntheses of 2H-1,3-oxazines and 1H-pyrrol-3(2H)-ones via temperature-dependent Rh(II)-carbenoid-mediated 2H-azirine ring expansion” Tetrahedron 2014, 70(21), 3377-3384 DOI: 10.1016/j.tet.2014.03.101 | The Rh(II)-catalyzed reaction of 2-carbonyl-substituted 2H-azirines with ethyl 2-cyano-2-diazoacetate or 2-diazo-3,3,3-trifluoropropionate provides an easy access to 2H-1,3-oxazines and 1H-pyrrol-3(2H)-ones. These compounds can be selectively prepared from the same starting material using temperature as the only varied parameter. | Bruker Avance 400 and Bruker DPX-300 NMR spectrometers. Structures of some compounds verified by 1H and 13C NMR, using CDCl3 and DMSO-d6 as solvents. |
| 42 | 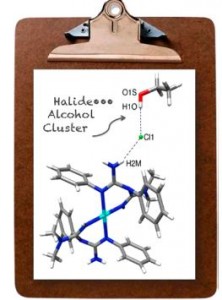 P.V. Gushchin, M.L. Kuznetsov, M. Haukka, V.Yu. Kukushkin, “Anionic halide…alcohol clusters in the solid state” J. Phys. Chem. A, 2014, 118, 9529-9539 DOI: 10.1021/jp506256a | The cationic (1,3,5-triazapentadiene)PtII complexes [1](Cl)2, [2](Cl)2, [3](Br)2, and [4](Cl)2, were crystallized from ROH-containing systems (R = Me, Et) providing alcohol solvates. Properties of the Cl−•••HO(Me) H-bond in [1][(Cl)2(MeOH)2] were analyzed using theoretical DFT methods. | Bruker Avance 400WB NMR spectrometer. Solid-state 1H NMR spectroscopy was used to study hydrogen bonding. |
| 43 | 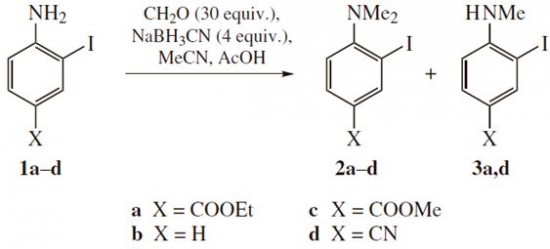 A.E. Kulyashova, E.V. Mikheeva, N.A. Danilkina, I.A. Balova, «Synthesis of 2-(buta-1,3-diynyl)-N,N-dimethylanilines using reductive methylation step» Mendeleev Commun., 2014, 24, 102-104 DOI: 10.1016/j.mencom.2014.03.013 | Synthesis of 2-(buta-1,3-diynyl)-N,N-dimethylanilines based on reductive methylation of ortho-iodoanilines using CH2O–NaBH3CN and coupling with terminal diacetylens was developed. Altered sequence including dimethylation of 2-(buta-1,3-diynyl)anilines was also successfully achieved and gave higher overall yields in the case of anilines without acceptor substituent in the ring. | 1D and 2D NMR spectra for structure elucidation of the studied compounds were measured on a Bruker Avance III 400 and Bruker Avance III 500 spectrometers. |
| 44 | 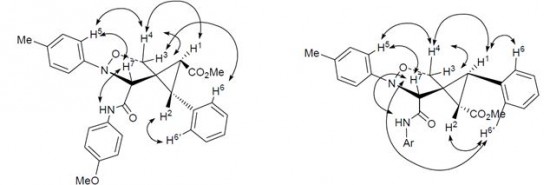 A.P. Molchanov, T.Q. Tran, A.V. Stepakov, G.L. Starova, R.R. Kostikov, «Regioselective cycloaddition of C-carbamoylnitrones to methyl (E)-2-(2-phenylcyclopropylidene)acetate and methyl (E)-2-methylidene-3-phenylcyclopropane-1-carboxylate» Russ. J. Org. Chem., 2014, 50, 78-82 (russ. 84-88) DOI: 10.1134/S1070428014010151 | N-Aryl-C-(arylcarbamoyl)nitrones regioselectively add to methyl 2-(2-phenylcyclopropylidene)- acetate and methyl 2-methylidene-3-phenylcyclopropanecarboxylate to give in each case two diastereoisomeric 5-oxa-6-azaspiro[2.4]heptane-4-carboxylates. | Bruker DPX-300 NMR spectrometer. The 1H and 13C NMR spectra were user for structure characterization. Stereochemistry was established by using 1H-1H NOESY NMR spectra |
| 45 | 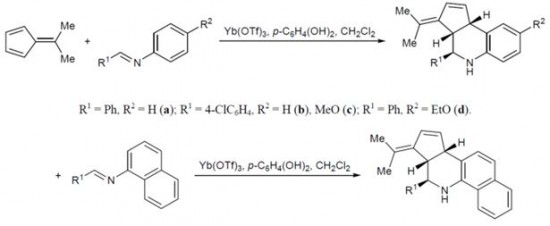 A.V. Stepakov, A.G. Larina, V.M. Boitsov, A.P. Molchanov, «Reaction of 6,6-dimethylfulvene with aromatic imines in the presence of Lewis acids» Russ. J. Org. Chem., 2014, 50, 50,389-393 (russ. 404-408) DOI: 10.1134/S1070428014030154 | Formal aza-Diels–Alder (Povarov) reaction of 6,6-dimethylfulvene with aromatic imines is described for the first time. | Bruker DPX-300 spectrometer. Structure of the synthesized compounds were verified by 1H and 13C NMR spectroscopy. |
| 46 | 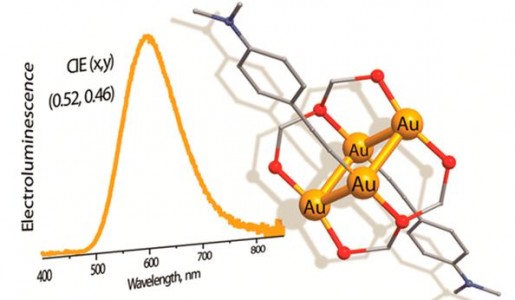 T. Minh Dau, Y.-A. Chen, A.J. Karttunen, E.V. Grachova, S.P. Tunik, K.-T. Lin, W.-Y. Hung, P.-T. Chou, T.A. Pakkanen, I.O. Koshevoy, “Tetragold(I) complexes: solution isomerization and tunable solid-state luminescence» Inorg. Chem. 2014, 53(24), 12720-31 DOI: 10.1021/ic501470v | The tetragold(I) clusters supported by the triphosphine ligand adopt two structural motifs and in solution exist as two isomeric species in slow chemical equilibria. In the solid state, the title complexes exhibit moderate-to-strong phosphorescence, exhibiting quantum yields up to 51%. Decent quantum efficiency allowed for the fabrication of an organic electroluminescent device, which has been achieved for the first time using polynuclear gold(I) compounds. | The solution 1H, 31P{1H}, 1H{31P}, and 1H-1H COSY NMR spectra were recorded on a Bruker Avance 400 spectrometer. Obtained NMR data show that the majority of the alkynyl clusters exist in a fluid medium as two isomeric species, being in slow chemical equilibria, which depends on the nature of the alkyne and solvent. |
| 47 | 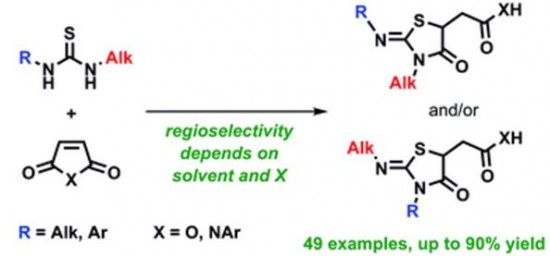 A.S. Pankova, M.A. Samartsev, I.A. Shulgin, P.R. Golubev, М.А. Кuznetsov, «Synthesis of thiazolidines via regioselective addition of unsymmetric thioureas to maleic acid derivatives» RSC Advances, 2014, 4, 51780-51786 DOI: 10.1039/c4ra07840c | A wide range of unsymmetric thioureas has been studied in reaction with N-arylmaleimides and maleic anhydride. The regioselectivity of the addition depends not only on steric factors but on both solvent polarity and type of maleic acid derivative (imide or anhydride). The general regularities have been established providing practical guidelines to control the reaction result. | Bruker DPX-300, Bruker Avance 400 and Bruker Avance 500 NMR spectrometers. The unequivocal structural assignment of all products has been done using 1H and 13C NMR spectroscopy including 15N-1H HMBC experiments. |