R.O. Iakovenko, A.N. Kazakova, V.M. Muzalevskiy, A.Yu. Ivanov, I.A. Boyarskaya, A. Chicca, V. Petrucci, J. Gertsch, M. Krasavin, G.L. Starova, A.A. Zolotarev, M.S. Avdontceva, V.G. Nenajdenko, A.V. Vasilyev
“Reactions of CF3-enones with arenes under superelectrophilic activation: a pathway to trans-1,3-diaryl-1-CF3-indanes, new cannabinoid receptor ligands”
Org. Biomol. Chem., 2015, 13, 8827-8842
DOI:10.1039/c5ob01072a
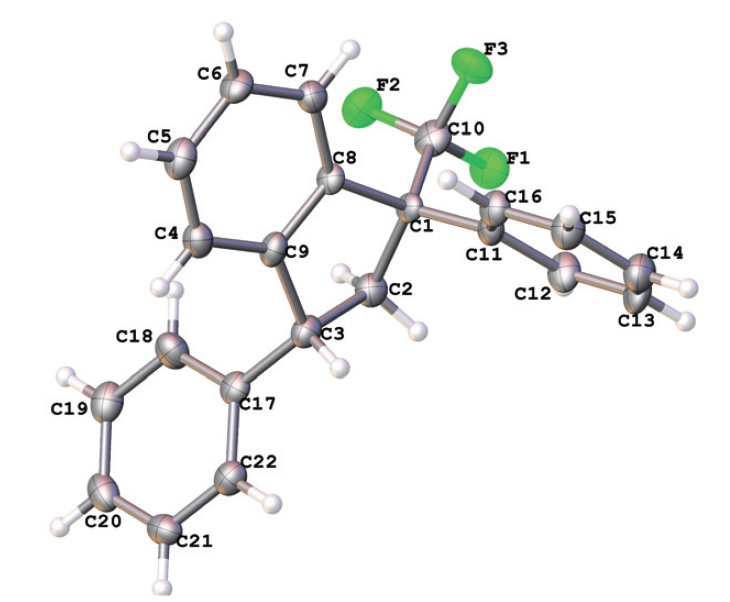
4-Aryl-1,1,1-trifluorobut-3-en-2-ones ArCH=CHCOCF3 (CF3-enones) react with arenes in excess of Brønsted superacids (TfOH, FSO3H) to give, stereoselectively, trans-1,3-diaryl-1-trifluoromethyl indanes in 35–85% yields. The reaction intermediates, the O-protonated ArCH=CHC(OH+)CF3 and the O,C-diprotonated ArHC+CH2C(OH+)CF3 species, have been studied by means of 1H, 13C, 19F NMR, and DFT calculations. Both types of the cations may participate in the reaction, depending on their electrophilicity and electron-donating properties of the arenes. The formation of CF3-indanes is a result of cascade reaction of protonated CF3-enones to form chemo-, regio- and stereoselectively three new C–C bonds. The obtained trans-1,3-diaryl-1-trifluoromethyl indanes were investigated as potential ligands for cannabinoid receptors CB1 and CB2 types. The most potent compound showed sub-micromolar affinity for both receptor subtypes with a 6-fold selectivity toward the CB2 receptor with no appreciable cytotoxicity toward SHSY5Y cells.