M. Promzeleva, T.V. Volkova, A.N. Proshin, O.I. Siluykov, A. Mazur, P.M. Tolstoy, S.P. Ivanov, F. Kamilov, I.V. Terekhova
“Improved biopharmaceutical properties of oral formulations of 1,2,4-thiadiazole derivative with cyclodextrins: in vitro and in vivo evaluation”
ACS Biomater. Sci. Eng, 2018, 4(2), 491-501
DOI: 10.1021/acsbiomaterials.7b00887
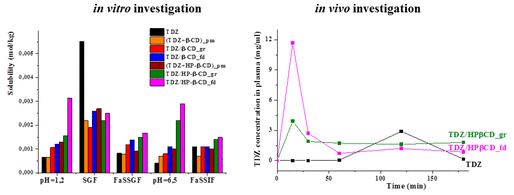
The synthesized 1,2,4-thiadiazole derivative displaying biological activity has low aqueous solubility and dissolution rate. Novel oral formulations of thiadiazole with β- and hydroxypropyl-β-cyclodextrins were obtained by grinding and freeze-drying methods with the purpose to improve the aqueous solubility. Complex formation of 1,2,4-thiadiazole derivative with cyclodextrins was confirmed by means of solid-state 13C MAS CP/TOSS NMR. Solubility, dissolution rate and permeability of the solid inclusion complexes were evaluated in different biorelevant media (SGF, FaSSGF, FaSSIF) simulating the conditions in the gastrointestinal tract. It was demonstrated that the content of biorelevant media affects the properties of the inclusion complexes. In particular, solubilizing effect of cyclodextrins became less pronounced when the micelles of taurocholic acid and lecithin are formed in the dissolution media. The inclusion of thiadiazole into cyclodextrin cavity is in competition with its partitioning into the micelles and this should be taken into account when the in vivo behavior is predicted. The results of in vitro and in vivo experiments were found to be in agreement and showed the highest solubility, dissolution rate and bioavailability of the freeze-dried complexes of thiadiazole with hydroxypropyl-β-cyclodextrin. These complexes can be proposed as more effective dosage forms for oral administration.