A. M. Afanasenko, D. V. Boyarskaya, I. A. Boyarskaya, T. G. Chulkova, Y. M. Grigoriev, I. E. Kolesnikov, M. S. Avdontceva, T. L. Panikorovskii, A. I. Panin, A. N. Vereshchagin, M. N. Elinson
“Structures and photophysical properties of 3,4-diaryl-1H-pyrrol-2,5-diimines and 2,3-diarylmaleimides”
Journal of Molecular Structure, 2017, 1146, 554–561
DOI: 10.1016/j.molstruc.2017.06.048
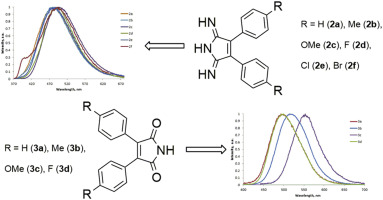
Structural features of 3,4-diaryl-1H-pyrrol-2,5-diimines and their derivatives have been studied by molecular spectroscopy techniques, single-crystal X-ray diffraction, and DFT calculations. According to the theoretical calculations, the diimino tautomeric form of 3,4-diaryl-1H-pyrrol-2,5-diimines is more stable in solution than the imino-enamino form. We also found that the structurally related 2,3-diarylmaleimides exist in the solid state in the dimeric diketo form. 3,4-Diaryl-1H-pyrrol-2,5-diimines and 2,3-diarylmaleimides exhibit fluorescence in the blue region of the visible spectrum. The fluorescence spectra have large Stokes shifts. Aryl substituents at the 3,4-positions of 1H-pyrrol-2,5-diimine do not significantly affect fluorescence properties. The insertion of donor substituents into 2,3-diarylmaleimides leads to bathochromic shift of emission bands with hyperchromic effect.