D.S. Bolotin, A.S. Novikov, V.K. Burianova, M.Ya. Demakova, E.A. Daines, С. Pretorius, P. Mokolokolo, A. Roodt, N.A. Bokach, M.S. Avdontceva, A.P. Zhdanov, K.Yu. Zhizhin, N.T. Kuznetsov, V.Yu. Kukushkin
“Nucleophilicity of oximes based upon addition to а nitrilium closo-decaborate cluster”
Organometallics, 2016, 35, 3612-3623
DOI:10.1021/acs.organomet.6b00678
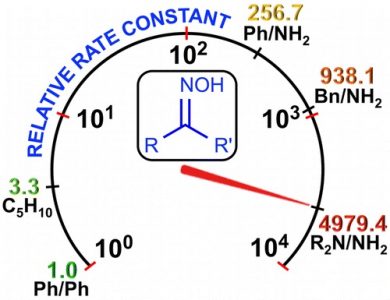
Three types of oxime species, i.e., 4-morpholylcarbamidoxime (hydroxyguanidine), phenylacetamidoxime and benzamidoxime (amidoximes), and cyclohexanone oxime and benzophenone oxime (ketoximes), react at room temperature with the 2-nitrilium closo-decaborate clusters, leading to 2-iminium closo-decaborates (14 examples; 57–94%). These species were characterized by ICPMS-based boron elemental analysis, HRESI–-MS, molar conductivity, IR, 1H{11B}, and 11B{1H} NMR spectroscopies, and additionally by single-crystal X-ray diffraction (for six compounds). On the basis of kinetic data, ΔH⧧, ΔS⧧, and ΔG⧧ of the additions were determined, showing a 4 order-of-magnitude decrease in reactivity from the hydroxyguanidine to the aromatic ketoxime as entering nucleophiles. The results of DFT calculations indicate that the mechanism for these reactions is stepwise and is realized through the formation of the orientation complex of the nitrone form, R2R3C═N+(H)O–, of oximes with [B10H9NCEt]−, giving further an acyclic intermediate (the rate-determining step), followed by proton migration, leading to the addition product. The calculated overall activation barrier for these transformations is consistent with the experimental kinetic observations. This work provides, for the first time, a broad nucleophilicity series of oximes, which is useful to control various nucleophilic additions of oxime species.