S.A. Kras’ko, S.S. Zlotskii, V.P. Boyarskii
“Comparative Activity of Aryl, Alkyl, and Cycloalkyl Halides in the Suzuki Reaction Catalyzed with Acyclic Diaminocarbene Complex of Palladium”
Russ. J. Gen. Chem., 2016, 85(11), 2541-2546
DOI:10.1134/S1070363215110079
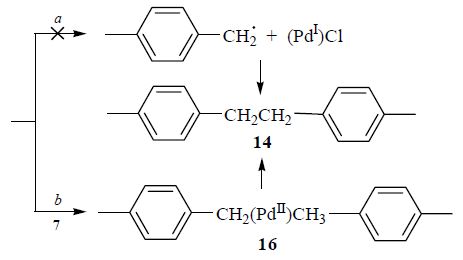
Relative activity of halogenated arenes, alkanes, and alkanes in the Suzuki reaction catalyzed with acyclic diaminocarbene complex of palladium has been investigated. Under all the investigated conditions, 4-iodoanisole has been more active than the alkyl halides. The reaction with 4-methyl-1-(chloromethyl)benzene has afforded the target 4-methyl-1-(phenylmethyl)benzene along with significant amount of by-products; other alkyl and cycloalkyl halides do not participate into the cross-coupling reaction. Ethanol has been found the most suitable solvent for the reaction. The reaction in acetonitrile provides noticeable yield of the products only in the presence of polyethylene glycol and water.