Archive for March 10, 2016
Excursion-lecture for 3rd year students (part 2)
Inorg. Chem. Comm. 2015, 61, 21-23
V.A. Rassadin, A.A. Yakimanskiy, E.V. Eliseenkov, V.P. Boyarskiy
“Synthesis of acyclic diaminocarbene palladium complex featuring triethoxysilane moiety”
Inorg. Chem. Comm., 2015, 2015, 61, 21-23
DOI:10.1016/j.inoche.2015.08.008
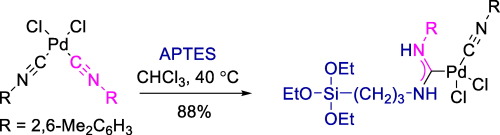
The first example of an acyclic diaminocarbene palladium complex, viz. cis-[PdCl2(CN(2,6-Me2C6H3)){C(NH(2,6-Me2C6H3)) = NH(CH2)3Si(OEt)3}], featuring the triethoxysilane moiety is described. The complex was generated from bis(o-xylylisocyanide)palladium dichloride and (3-aminopropyl)triethoxysilane under mild conditions and isolated in 88% yield. The target compound is stable at RT either in the solid state or in CDCl3 or CD3OD solutions within several months.
Febrary
Total in Febrary 1749 service applications were carried out.
All together measured:
- 1680 1H spectra
- 341 13C spectra
- 150 DEPT spectra
- 24 COSY spectra
- 15 NOESY spectra
- 73 31P spectra
- 58 19F spectra
206 applications were carried out.
Inorg. Chem., 2015, 54, 4039-4046
D.S. Bolotin, M.Ya. Demakova, A.S. Novikov, M.S. Avdontceva, M.L. Kuznetsov, N.A. Bokach, V.Yu. Kukushkin
“Bifunctional Reactivity of Amidoximes Observed upon Nucleophilic Addition to Metal-Activated Nitriles”
Inorg. Chem., 2015, 54, 4039-4046
DOI:10.1021/acs.inorgchem.5b00253
Treatment of the aromatic nitrile complexes trans-[PtCl2(RC6H4CN)2] with the aryl amidoximes p-R′C6H4C(NH2)═NOH, followed by addition of 1 equiv of AgOTf and then 5 equiv of Et3N, leads to the chelates [PtCl{HN═C(RC6H4)ON═C(C6H4R′-p)NC(RC6H4)═NH}] (15 examples; yields 71−88% after column chromatography) derived from the platinum(II)-mediated coupling between metal-activated nitriles and amidoximes. The combined experimental and theoretical results indicate that the coupling with the nitrile ligands involves both the HON and monodeprotonated NH2 groups of the amidoximes.
Org. Lett. 2015, 17, 3502-3505
V.A. Rassadin, V.P. Boyarskiy, V.Yu. Kukushkin
“Facile Gold-Catalyzed Heterocyclization of Terminal Alkynes and Cyanamides Leading to Substituted 2-Amino-1,3-Oxazoles”
Org. Lett., 2015, 17, 3502-3505
DOI:10.1021/acs.orglett.5b01592
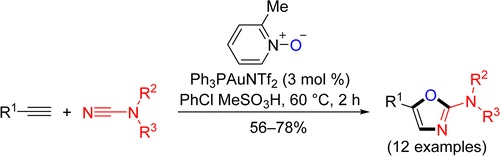
Facile gold-catalyzed heterocyclization based upon intermolecular trapping of the generated α-oxo gold carbenes with various cyanamides R2R3NCN (R2/R3 = Alk/Alk, −(CH2)2O(CH2)2–, Ar/Ar, Ar/H) has been developed. In most cases, 2-amino-1,3-oxazoles functionalized at the nitrogen atom as well as at the fifth position of the heterocyclic ring (12 examples) were isolated in good to moderate yields.


