D. Dar’In, O. Bakulina, M. Chizhova, M. Krasavin
“New Heterocyclic Product Space for the Castagnoli-Cushman Three-Component Reaction”
Organic Lett., 2015, 17, 3930-3933
DOI:10.1021/acs.orglett.5b02014
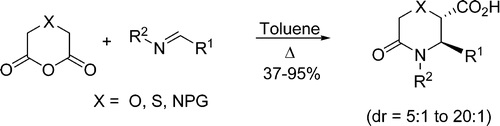
Significant expansion of heterocyclic product space accessible by the Castagnoli–Cushman reaction (CCR) has been achieved via the use of glutaric anhydride analogues containing endocyclic substitutions with oxygen, nitrogen, and sulfur. Incorporation of these heteroatoms in the anhydride’s backbone results in enhanced reactivity and generally lower temperatures that are required for the reactions to go to completion. These findings are particularly significant in light of the CCR recently recognized as an efficient tool for lead-oriented synthesis.