E.A. Valishina, M.F.C. Guedes da Silva, M.A. Kinzhalov, S.A. Timofeeva, T.M. Buslaeva, M. Haukka, A.J.L. Pombeiro, V.P. Boyarskiy, V.Yu. Kukushkin, K.V. Luzyanin
“Palladium-ADC complexes as efficient catalysts in copper-free and room temperature Sonogashira coupling”
J. Molec. Catalysis , 2014, 395, 162-171
DOI:10.1016/j.molcata.2014.08.018
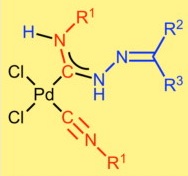
The metal-mediated coupling between cis-[PdCl2(CNR1)2] [R1 = cyclohexyl (Cy) 1, t-Bu 2, 2,6-Me2C6H3 (Xyl) 3, 2-Cl-6-MeC6H3 4] and hydrazones H2NN=CR2R3 [R2, R3 = Ph 5; R2, R3 = C6H4(OMe-4) 6; R2/R3 = 9-fluorenyl 7; R2 = H, R3 = C6H4(OH-2) 8] provided carbene complexes cis-[PdCl2{C(N(H)N=CR2R3)=N(H)R1}(CNR1)] (9–24) in good (80–85%) yields. Complexes 9–24 showed high activity [yields up to 99%, and turnover numbers (TONs) up to 3.7 × 104] in the Sonogashira coupling of various aryl iodides with a range of substituted aromatic alkynes without the need of copper co-catalyst. The catalytic procedure runs at 80 °C for 1 h in EtOH using K2CO3 as a base. No formation of homocoupling or acetylene decomposition products was observed. Designed copper-free Sonogashira system can also run at room temperature giving target products with yields up to 87% and TONs up to 87.