A. Miroslavov, Y. Polotskii, V. Gurzhiy, A. Ivanov, A. Lumpov, M. Tyupina, G. Sidorenko, P. Tolstoy, D. Maltsev, D. Suglobov
“Technetium and Rhenium Pentacarbonyl Complexes with C2 and C11 ω-Isocyanocarboxylic Acid Esters”
Inorg. Chem. 2014, Article ASAP
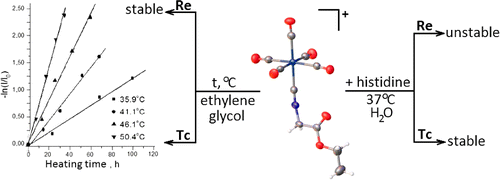
Technetium(I) and rhenium(I) pentacarbonyl complexes with ethyl 2-isocyanoacetate and methyl 11-isocyanoundecanoate, [M(CO)5(CNCH2COOEt)]ClO4 (M = Tc (1) and Re (2)) and [M(CO)5(CN(CH2)10COOMe)]ClO4 (M = Tc (3) and Re (4)), were prepared and characterized by IR, 1H NMR, and 13C{1H} NMR spectroscopy. The crystal structures of 1 and 2 were determined using single-crystal X-ray diffraction. The kinetics of thermal decarbonylation of technetium complexes 1 and 3 in ethylene glycol was studied by IR spectroscopy. The rate constants and activation parameters of this reaction were determined and compared with those for [Tc(CO)6]+. It was found that rhenium complexes 2 and 4 were stable with respect to thermal decarbonylation. Histidine challenge reaction of complexes 1 and 2 in phosphate buffer was examined by IR spectroscopy. In the presence of histidine, the rhenium pentacarbonyl isocyanide complex partially decomposes to form an unidentified yellow precipitate. Technetium analogue 1 is more stable under these conditions.