J. Guo, P. M. Tolstoy, B. Koeppe, N. S. Golubev, G. S. Denisov, S. N. Smirnov, H.-H. Limbach
“Hydrogen Bond Geometries and Proton Tautomerism of Homo-Conjugated Anions of Carboxylic Acids Studied via H/D Isotope Effects on 13C NMR Chemical Shifts”
J. Phys. Chem. A 2012, ASAP.
DOI: 10.1021/jp304943h.
Abstract:
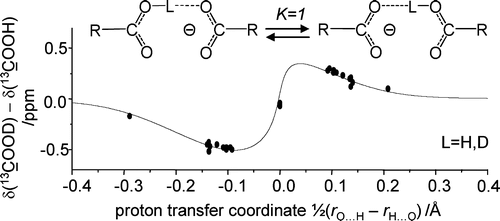
Ten formally symmetric anionic OHO hydrogen bonded complexes, modeling Asp/Glu amino acid side chain interactions in nonaqueous environment (CDF3/CDF2Cl solution, 200–110 K) have been studied by 1H, 2H, and 13C NMR spectroscopy, i.e. intermolecularly H-bonded homoconjugated anions of acetic, chloroacetic, dichloroacetic, trifluoroacetic, trimethylacetic, and isobutyric acids, and intramolecularly H-bonded hydrogen succinate, hydrogen rac-dimethylsuccinate, hydrogen maleate, and hydrogen phthalate. In particular, primary H/D isotope effects on the hydrogen bond proton signals as well as secondary H/D isotope effects on the 13C signals of the carboxylic groups are reported and analyzed. We demonstrate that in most of the studied systems there is a degenerate proton tautomerism between O–H···O– and O–···H–O structures which is fast in the NMR time scale. The stronger is the proton donating ability of the acid, the shorter and more symmetric are the H-bonds in each tautomer of the homoconjugate. For the maleate and phthalate anions exhibiting intramolecular hydrogen bonds, evidence for symmetric single well potentials is obtained. We propose a correlation between H/D isotope effects on carboxylic carbon chemical shifts and the proton transfer coordinate, q1 = 1/2(rOH – rHO), which allows us to estimate the desired OHO hydrogen bond geometries from the observed 13C NMR parameters, taking into account the degenerate proton tautomerism.