L.D. Funt, O.A. Tomashenko, A.F. Khlebnikov M.S. Novikov, A.Yu. Ivanov
“Synthesis, Transformations of Pyrrole- and 1,2,4-Triazole-Containing Ensembles, and Generation of Pyrrole-Substituted Triazole NHC”
J. Org. Chem., 2016, 81, 11210-11221
DOI:10.1021/acs.joc.6b02200
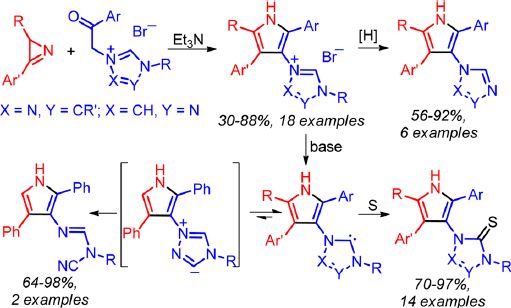
Unprecedented pyrrole- and 1,2,4-triazole-containing ensembles, substituted 1-(1H-pyrrol-3-yl)-4H-1,2,4-triazol-1- ium bromides and 4-(1H-pyrrol-3-yl)-1H-1,2,4-triazol-4-ium bromides, were prepared from 2H-azirines and triazolium phenacyl bromides using a simple procedure. N-(1H-Pyrrol-3-yl)-N′-benzyltriazolium bromides undergo reductive debenzylation on Pd/C to give substituted 1-(1H-pyrrol-3-yl)-4H-1,2,4-triazoles and 4-(1H-pyrrol-3-yl)-1H-1,2,4-triazoles in high yields. Betaines (triazoliumylpyrrolides) and pyrrolyltriazole NHCs, which are possible products of dehydrobromination of pyrrolyltriazolium salts, have comparable thermodynamic stabilities in nonpolar solvents according to calculations at the DFT B3LYP/6-31G(d) level. The carbene forms can be easily trapped by the reaction of salts with base in the presence of sulfur. The corresponding 1- and 4-(1H-pyrrol-3-yl)-1H-1,2,4-triazole-5(4H)-thiones are formed in high yields. In the absence of sulfur as a trap, the opening of the triazole ring occurs with the formation of derivatives of N-cyanoformimidamide. According to the DFT calculations the latter is most probably formed via a pyrrolyltriazoliumide intermediate, which is the minor component of the equilibrium triazoliumylpyrrolide−pyrrolyltriazole NHC−pyrrolyltriazoliumide. Blocking of the pyrrolyltriazoliumide intermediate formation, by introduction of a substituent at the 3-position of the triazole ring, made it possible to generate the first pyrrole-substituted triazole NHC.