M.V. Sorokina, A.S. Pankova, M.A. Kuznetsov
“Oxidative Aminoaziridination of 2-Vinylfuran Derivatives as an Approach to Hexa-2,5-diene-1,4-dione Monohydrazones”
Asian J. Org. Chem., 2016, 5, 389-398
DOI:10.1002/ajoc.201500460
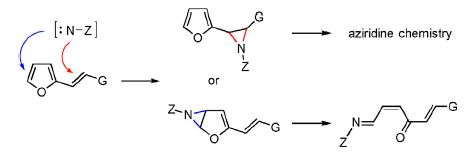
The oxidative addition of N-aminophthalimide to substituted 2-vinylfurans provides monophthaloylhydrazones of (2Z)-hexa-2,5-diene-1,4-dione derivatives instead of 2-furylaziridines by proceeding through an aziridination of the endocyclic furan C=C bond followed by a regio- and stereoselective rearrangement of the bicyclic intermediate. The formation of stable aziridines can then occur through the aziridination of the (E)-C=C bond of these phthaloylhydrazones. Detailed structure elucidations and mechanistic considerations are provided.