S. Saulnier, A.A. Golovanov, A.Yu. Ivanov, I.A. Boyarskaya, A.V. Vasilyev
“Transformations of Conjugated Enynones in the Superacid CF3SO3H. Synthesis of Butadienyl Triflates, Indanones, and Indenes”
J. Org. Chem., 2016, 81, 1967-1980
DOI:10.1021/acs.joc.5b02785
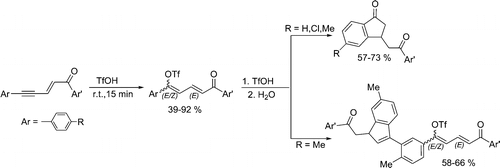
Conjugated 1,5-diarylpent-2-en-4-yn-1-ones add the superacid CF3SO3H to the acetylenic bond with formation of the corresponding butadienyl triflates. Under superacidic reaction conditions, these triflates are transformed into indanone or indene derivatives depending on which substituents on the aromatic ring are conjugated with the butadiene fragment. In a less acidic system (10% vol pyridine in CF3SO3H) only the formation of butadienyl triflates takes place. Cationic reaction intermediates were studied by means of NMR and DFT calculations.