Yu.B. Koptelov, D.O. Antuganov, A.P. Molchanov, R.R. Kostikov
“Steric Hindrances to the Cycloaddition of (Z)-1-Arylmethylidene-5,5-dimethyl-3-oxopyrazolidin-1-ium-2-ides to N-Arylmaleimides”
Russ. J. Org. Chem., 2015, 7, 972-981
DOI:10.1134/S1070428015070143
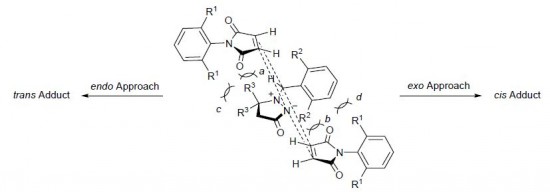
Sterically hindered cycloaddition of (Z)-1-arylmethylidene-5,5-dimethyl-3-oxopyrazolidin-1-ium-2-ides to 4-mono- and 2,6-disubstituted N-arylmaleimides requires prolonged heating (40–60 h) at ~150–155°C and yields mixtures of diastereoisomeric cycloadducts. The observed diastereoselectivity is determined by both electronic and steric interactions, depending on the nature and position of substituents in the azomethine imine and maleimide. The reactions of (Z)-1-(2,6-dichlorobenzylidene)-5,5-dimethyl-3-oxopyrazolidin-1-ium-2-ide with 4-substituted N-arylmaleimides give mainly the corresponding cis adducts as a result of preferential exo attack by the dipolarophile, whereas trans adducts predominate in the cycloaddition of (Z)-1-(4-X-benzylidene)-5,5-dimethyl-3-oxopyrazolidin-1-ium-2-ide and (Z)-1-(2,6-dichlorobenzylidene) -5,5-dimethyl-3-oxopyrazolidin- 1-ium-2-ide to 2,6-disubstituted N-arylmaleimides.