S.N. Britvin, A. Lotnyk
“Water-Soluble Phosphine Capable of Dissolving Elemental Gold: The Missing Link between 1,3,5-Triaza-7-phosphaadamantane (PTA) and Verkade’s Ephemeral Ligand”
J. Am. Chem. Soc., 2015, accepted
DOI:10.1021/jacs.5b01851
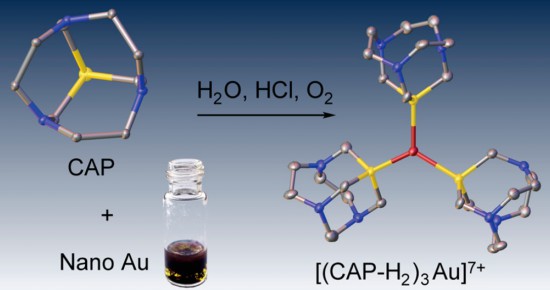
We herein describe a tricyclic phosphine with previously unreported tris(homoadamantane) cage architecture. That water-soluble, air- and thermally stable ligand, 1,4,7-triaza-9-phosphatricyclo[5.3.2.14,9]tridecane (hereinafter referred to as CAP) exhibits unusual chemical behavior toward gold and gold compounds: it readily reduces Au(III) to Au(0), promotes oxidative dissolution of nanocrystalline gold(0) with the formation of water-soluble trigonal CAP−Au(I) complexes, and displaces cyanide from [Au(CN)2]− affording triangular [Au(CAP)3]+ cation. From the stereochemical point of view, CAP can be regarded as an intermediate between 1,3,5-triaza-7-phosphaadamantane (PTA) and very unstable aminophosphine synthesized by Verkade’s group: exahydro-2a,4a,6a-triaza-6bphosphacyclopenta[cd]pentalene. The chemical properties of CAP are likely related to its anomalous stereoelectronic profile: combination of strong electron-donating power (Tolman’s electronic parameter 2056.8 cm−1) with the low steric demand (coneangle of 109°). CAP can be considered as macrocyclic counterpart of PTA with the electron-donating power approaching that of strongest known phosphine electron donors such as P(t-Bu)3 and PCy3. Therefore, CAP as sterically undemanding and electronrich ligand populates the empty field on the stereoelectronic map of phosphine ligands: the niche between the classic tertiary phosphines and the sterically undemanding aminophosphines.