Alena S. Pankova, Mikhail A. Kuznetsov
“Synthesis and thermal transformations of spiro-fused N-phthalimidoaziridines”
Tetrahedron Letters, 2014, In Press
DOI: 10.1016/j.tetlet.2014.03.014
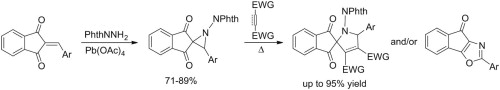
Oxidation of N-aminophthalimide in the presence of 2-arylideneinden-1,3-diones with electron-withdrawing substituents gives the corresponding 3-aryl-1-phthalimidospiro[aziridine-2,2’-indene]-1’,3’-diones in good yields. Heating these aziridines with standard dipolarophiles (N-phenylmaleimide, dimethyl acetylenedicarboxylate, maleate and fumarate) leads, in most cases, to spiro[inden-2,2’-pyrrole] derivatives as products of 1,3-dipolar cycloaddition of the intermediate azomethine ylides with up to 70-95% yields in the case of N-phenylmaleimide. As is typical for 2-acylaziridines, the competing rearrangement into 2-aryl-4H-indeno[2,1-d][1,3]oxazol-4-ones prevails for less active dipolarophiles. Increasing the electron-releasing properties of the 3-aryl ring allows the observation of the push-pull effect of electron-donating and electron-withdrawing substituents on the ease of the three-membered ring-opening.